Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade
- PMID: 19864584
- PMCID: PMC2796271
- DOI: 10.1523/JNEUROSCI.3018-09.2009
Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade
Abstract
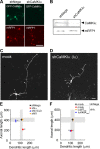
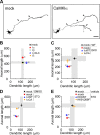
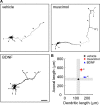
Ca(2+) signaling plays important roles during both axonal and dendritic growth. Yet whether and how Ca(2+) rises may trigger and contribute to the development of long-range cortical connections remains mostly unknown. Here, we demonstrate that two separate limbs of the Ca(2+)/calmodulin-dependent protein kinase kinase (CaMKK)-CaMKI cascades, CaMKK-CaMKIalpha and CaMKK-CaMKIgamma, critically coordinate axonal and dendritic morphogenesis of cortical neurons, respectively. The axon-specific morphological phenotype required a diffuse cytoplasmic localization and a strikingly alpha-isoform-specific kinase activity of CaMKI. Unexpectedly, treatment with muscimol, a GABA(A) receptor agonist, selectively stimulated elongation of axons but not of dendrites, and the CaMKK-CaMKIalpha cascade critically mediated this axonogenic effect. Consistent with these findings, during early brain development, in vivo knockdown of CaMKIalpha significantly impaired the terminal axonal extension and thereby perturbed the refinement of the interhemispheric callosal projections into the contralateral cortices. Our findings thus indicate a novel role for the GABA-driven CaMKK-CaMKIalpha cascade as a mechanism critical for accurate cortical axon pathfinding, an essential process that may contribute to fine-tuning the formation of interhemispheric connectivity during the perinatal development of the CNS.
Figures




Comment in
-
CaMKK-CaMKI signaling pathways differentially control axon and dendrite elongation in cortical neurons.J Neurosci. 2010 Feb 24;30(8):2807-9. doi: 10.1523/JNEUROSCI.5984-09.2010. J Neurosci. 2010. PMID: 20181577 Free PMC article. Review. No abstract available.
References
-
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. - PubMed
-
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. - PubMed
-
- Bito H, Takemoto-Kimura S. Ca2+/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
