Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes
- PMID: 19864630
- PMCID: PMC2770008
- DOI: 10.1073/pnas.0906524106
Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes
Abstract
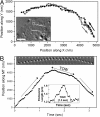
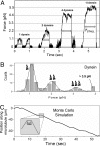
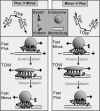
Intracellular transport is interspersed with frequent reversals in direction due to the presence of opposing kinesin and dynein motors on organelles that are carried as cargo. The cause and the mechanism of reversals are unknown, but are a key to understanding how cargos are delivered in a regulated manner to specific cellular locations. Unlike established single-motor biophysical assays, this problem requires understanding of the cooperative behavior of multiple interacting motors. Here we present measurements inside live Dictyostelium cells, in a cell extract and with purified motors to quantify such an ensemble function of motors. We show through precise motion analysis that reversals during endosome motion are caused by a tug-of-war between kinesin and dynein. Further, we use a combination of optical trap-based force measurements and Monte Carlo simulations to make the surprising discovery that endosome transport uses many (approximately four to eight) weak and detachment-prone dyneins in a tug-of-war against a single strong and tenacious kinesin. We elucidate how this clever choice of dissimilar motors and motor teams achieves net transport together with endosome fission, both of which are important in controlling the balance of endocytic sorting. To the best of our knowledge, this is a unique demonstration that dynein and kinesin function differently at the molecular level inside cells and of how this difference is used in a specific cellular process, namely endosome biogenesis. Our work may provide a platform to understand intracellular transport of a variety of organelles in terms of measurable quantities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. - PubMed
-
- Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14:R525–R537. - PubMed
-
- Gross SP. Hither and yon: A review of bi-directional microtubule-based transport. Phys Biol. 2004;1:R1–R11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

