The role of DNA shape in protein-DNA recognition
- PMID: 19865164
- PMCID: PMC2793086
- DOI: 10.1038/nature08473
The role of DNA shape in protein-DNA recognition
Abstract
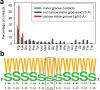
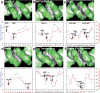
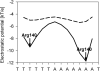
The recognition of specific DNA sequences by proteins is thought to depend on two types of mechanism: one that involves the formation of hydrogen bonds with specific bases, primarily in the major groove, and one involving sequence-dependent deformations of the DNA helix. By comprehensively analysing the three-dimensional structures of protein-DNA complexes, here we show that the binding of arginine residues to narrow minor grooves is a widely used mode for protein-DNA recognition. This readout mechanism exploits the phenomenon that narrow minor grooves strongly enhance the negative electrostatic potential of the DNA. The nucleosome core particle offers a prominent example of this effect. Minor-groove narrowing is often associated with the presence of A-tracts, AT-rich sequences that exclude the flexible TpA step. These findings indicate that the ability to detect local variations in DNA shape and electrostatic potential is a general mechanism that enables proteins to use information in the minor groove, which otherwise offers few opportunities for the formation of base-specific hydrogen bonds, to achieve DNA-binding specificity.
Figures


Comment in
-
Structural biology: DNA binding shapes up.Nature. 2009 Oct 29;461(7268):1225-6. doi: 10.1038/4611225a. Nature. 2009. PMID: 19865161 No abstract available.
References
-
- Garvie CW, Wolberger C. Recognition of specific DNA sequences. Mol Cell. 2001;8(5):937–946. - PubMed
-
- Travers AA. DNA conformation and protein binding. Annu Rev Biochem. 1989;58:427–452. - PubMed
-
- Shakked Z, et al. Determinants of repressor/operator recognition from the structure of the trp operator binding site. Nature. 1994;368(6470):469–473. - PubMed
-
- Lu XJ, Shakked Z, Olson WK. A-form conformational motifs in ligand-bound DNA structures. J Mol Biol. 2000;300(4):819–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

