PKA mediates constitutive activation of CFTR in human sweat duct
- PMID: 19865788
- PMCID: PMC2776937
- DOI: 10.1007/s00232-009-9205-1
PKA mediates constitutive activation of CFTR in human sweat duct
Abstract
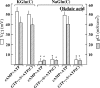
The cystic fibrosis transmembrane conductance regulator (CFTR) Cl(-) channels are constitutively activated in sweat ducts. Since phosphorylation-dependent and -independent mechanisms can activate CFTR, we sought to determine the actual mechanism responsible for constitutive activation of these channels in vivo. We show that the constitutively activated CFTR Cl(-) conductance (gCFTR) in the apical membrane is completely deactivated following alpha-toxin permeabilization of the basolateral membrane. We investigated whether such inhibition of gCFTR following permeabilization is due to the loss of cytoplasmic glutamate or due to dephosphorylation of CFTR by an endogenous phosphatase in the absence of kinase activity (due to the loss of kinase agonist cAMP, cGMP or GTP through alpha-toxin pores). In order to distinguish between these two possibilities, we examined the effect of inhibiting the endogenous phosphatase activity with okadaic acid (10(-8) M) on the permeabilization-induced deactivation of gCFTR. We show that okadaic acid (1) inhibits an endogenous phosphatase responsible for dephosphorylating cAMP but not cGMP or G protein-activated CFTR and (2) prevents deactivation of CFTR following permeabilization of the basolateral membrane. These results indicate that distinctly different phosphatases may be responsible for dephosphorylating different kinase-specific sites on CFTR. We conclude that the phosphorylation by PKA alone appears to be primarily responsible for constitutive activation of gCFTR in vivo.
Figures


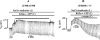
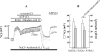

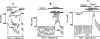

Similar articles
-
Functional interaction of CFTR and ENaC in sweat glands.Pflugers Arch. 2003 Jan;445(4):499-503. doi: 10.1007/s00424-002-0959-x. Epub 2002 Nov 21. Pflugers Arch. 2003. PMID: 12548396
-
ENaC activity requires CFTR channel function independently of phosphorylation in sweat duct.J Membr Biol. 2005 Sep;207(1):23-33. doi: 10.1007/s00232-005-0798-8. J Membr Biol. 2005. PMID: 16463140
-
Deactivation of CFTR-Cl conductance by endogenous phosphatases in the native sweat duct.Am J Physiol. 1996 Feb;270(2 Pt 1):C474-80. doi: 10.1152/ajpcell.1996.270.2.C474. Am J Physiol. 1996. PMID: 8779909
-
Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum.Biochim Biophys Acta. 1999 Dec 6;1461(2):275-83. doi: 10.1016/s0005-2736(99)00163-7. Biochim Biophys Acta. 1999. PMID: 10581361 Review.
-
Selective activation of cystic fibrosis transmembrane conductance regulator Cl- and HCO3- conductances.JOP. 2001 Jul;2(4 Suppl):212-8. JOP. 2001. PMID: 11875262 Review.
Cited by
-
CFTR-mediated Cl(-) transport in the acinar and duct cells of rabbit lacrimal gland.Curr Eye Res. 2012 Aug;37(8):671-7. doi: 10.3109/02713683.2012.675613. Epub 2012 May 11. Curr Eye Res. 2012. PMID: 22578307 Free PMC article.
-
Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction.J Biol Chem. 2012 Dec 28;287(53):44384-93. doi: 10.1074/jbc.M112.393488. Epub 2012 Oct 24. J Biol Chem. 2012. PMID: 23095755 Free PMC article.
-
Eccrine sweat gland development and sweat secretion.Exp Dermatol. 2015 Sep;24(9):644-50. doi: 10.1111/exd.12773. Epub 2015 Jul 14. Exp Dermatol. 2015. PMID: 26014472 Free PMC article. Review.
-
Role of CFTR in epithelial physiology.Cell Mol Life Sci. 2017 Jan;74(1):93-115. doi: 10.1007/s00018-016-2391-y. Epub 2016 Oct 6. Cell Mol Life Sci. 2017. PMID: 27714410 Free PMC article. Review.
-
The regulatory role of vasoactive intestinal peptide in lacrimal gland ductal fluid secretion: A new piece of the puzzle in tear production.Mol Vis. 2020 Dec 6;26:780-788. eCollection 2020. Mol Vis. 2020. PMID: 33311973 Free PMC article.
References
-
- Berger HA, Travis SM, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by specific protein kinases and protein phosphatases. J Biol Chem. 1993;268:2037–2047. - PubMed
-
- French PJ, Bijman J, Edixhoven M, Vaandrager AB, Scholte BJ, Lohmann SM, Nairn AC, De Jonge HR. Isotype-specific activation of cystic fibrosis transmembrane conductance regulator-chloride channels by cGMP-dependent protein kinase II. J Biol Chem. 1995;270:26626–26631. doi: 10.1074/jbc.270.44.26626. - DOI - PubMed

