The drug transporter-metabolism alliance: uncovering and defining the interplay
- PMID: 19874004
- PMCID: PMC2796475
- DOI: 10.1021/mp900253n
The drug transporter-metabolism alliance: uncovering and defining the interplay
Abstract
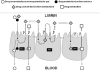
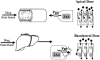
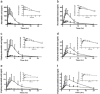
Two decades ago the importance of transporter-enzyme interplay and its effects on drug bioavailability and hepatic disposition were first recognized. Here we review the history of uncovering and defining this interplay with a primary emphasis on studies from our laboratory. We review the early 1990s oral bioavailability studies that found that the highly lipophilic, poorly water-soluble cyclosporine formulation on the market at that time did not have an absorption problem, but rather a gut metabolism problem. This led to studies of the interactive nature of CYP3A and P-glycoprotein in the intestine, and investigations of this interplay using cellular systems and isolated perfused rat organ studies. Studies investigating uptake transporter-enzyme interactions using cellular, perfused rat liver and intact rats are reviewed, followed by the human transporter-enzyme interaction studies. Work characterizing the rate limiting processes in the drug transporter-metabolism alliance is then addressed, ending with a review of areas of the interplay that require further studies and analysis.
Figures

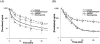


References
-
- Wacher VJ, Salphati L, Benet LZ. Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv. Drug Del. Rev. 1996;20:99–112. - PubMed
-
- Benet LZ, Wu C-Y, Hebert MF, Wacher VJ. Intestinal drug metabolism and antitransport processes: a potential paradigm shift in oral drug delivery. J. Control. Rel. 1996;39:139–143.
-
- Gupta SK, Manfro RC, Tomlanovich SJ, Gambertoglio JG, Garovoy MR, Benet LZ. Effect of food on the pharmacokinetics of cyclosporine in healthy subjects following oral and intravenous administration. J. Clin. Pharmacol. 1990;30:643–653. - PubMed
-
- Gupta SK, Benet LZ. Absorption kinetics of cyclosporine in healthy volunteers. Biopharm. Drug Dispos. 1989;10:591–596. - PubMed
-
- Gupta SK, Benet LZ. High fat meals increase the clearance of cyclosporine. Pharm. Res. 1990;7:46–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

