Kinetics of stop codon recognition by release factor 1
- PMID: 19874047
- PMCID: PMC2789991
- DOI: 10.1021/bi901577d
Kinetics of stop codon recognition by release factor 1
Abstract
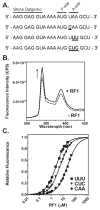
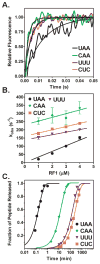
Recognition of stop codons by class I release factors is a fundamental step in the termination phase of protein synthesis. Since premature termination is costly to the cell, release factors have to efficiently discriminate between stop and sense codons. To understand the mechanism of discrimination between stop and sense codons, we developed a new, pre-steady state kinetic assay to monitor the interaction of RF1 with the ribosome. Our results show that RF1 associates with similar association rate constants with ribosomes programmed with stop or sense codons. However, dissociation of RF1 from sense codons is as much as 3 orders of magnitude faster than from stop codons. Interestingly, the affinity of RF1 for ribosomes programmed with different sense codons does not correlate with the defects in peptide release. Thus, discrimination against sense codons is achieved with both an increase in the dissociation rates and a decrease in the rate of peptide release. These results suggest that sense codons inhibit conformational changes necessary for RF1 to stably bind to the ribosome and catalyze peptide release.
Figures
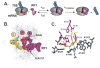
References
-
- Brenner S, Stretton AO, Kaplan S. Genetic code: the ‘nonsense’ triplets for chain termination and their suppression. Nature. 1965;206:994–998. - PubMed
-
- Konecki DS, Aune KC, Tate W, Caskey CT. Characterization of reticulocyte release factor. J Biol Chem. 1977;252:4514–4520. - PubMed
-
- Jorgensen F, Adamski FM, Tate WP, Kurland CG. Release factor-dependent false stops are infrequent in Escherichia coli. J Mol Biol. 1993;230:41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

