Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts
- PMID: 19874086
- PMCID: PMC3153381
- DOI: 10.1359/jbmr.090806
Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts
Abstract
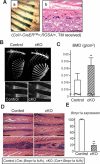
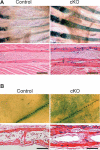
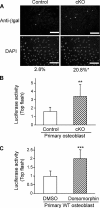
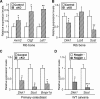
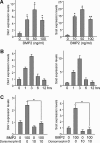
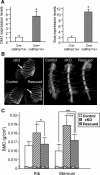
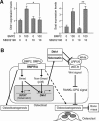
The bone morphogenetic protein (BMP) and Wnt signaling pathways both contribute essential roles in regulating bone mass. However, the molecular interactions between these pathways in osteoblasts are poorly understood. We recently reported that osteoblast-targeted conditional knockout (cKO) of BMP receptor type IA (BMPRIA) resulted in increased bone mass during embryonic development, where diminished expression of Sost as a downstream effector of BMPRIA resulted in increased Wnt/beta-catenin signaling. Here, we report that Bmpr1a cKO mice exhibit increased bone mass during weanling stages, again with evidence of enhanced Wnt/beta-catenin signaling as assessed by Wnt reporter TOPGAL mice and TOPFLASH luciferase. Consistent with negative regulation of the Wnt pathway by BMPRIA signaling, treatment of osteoblasts with dorsomorphin, an inhibitor of Smad-dependent BMP signaling, enhanced Wnt signaling. In addition to Sost, Wnt inhibitor Dkk1 also was downregulated in cKO bone. Expression levels of Dkk1and Sost were upregulated by BMP2 treatment and downregulated by Noggin. Moreover, expression of a constitutively active Bmpr1a transgene in mice resulted in the upregulation of both Dkk1 and Sost and partially rescued the Bmpr1a cKO bone phenotype. These effectors are differentially regulated by mitogen-activated protein kinase (MAPK) p38 because pretreatment of osteoblasts with SB202190 blocked BMP2-induced Dkk1 expression but not Sost. These results demonstrate that BMPRIA in osteoblasts negatively regulates endogenous bone mass and Wnt/beta-catenin signaling and that this regulation may be mediated by the activities of Sost and Dkk1. This study highlights several interactions between BMP and Wnt signaling cascades in osteoblasts that may be amenable to therapeutic intervention for the modification of bone mass density.
Copyright 2010 American Society for Bone and Mineral Research.
Figures
References
-
- Massague J. Receptors for the TGF-β family. Cell. 1992;69:1067–1070. - PubMed
-
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. - PubMed
-
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. - PubMed
-
- Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. - PubMed
-
- Keller S, Nickel J, Zhang JL, Sebald W, Mueller TD. Molecular recognition of BMP-2 and BMP receptor IA. Nat Struct Mol Biol. 2004;11:481–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

