Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors
- PMID: 19874199
- PMCID: PMC5524372
- DOI: 10.1359/jbmr.091036
Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors
Abstract
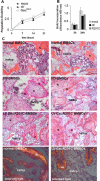
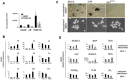
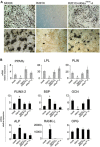
Human skeletal progenitors were engineered to stably express R201C mutated, constitutively active Gs alpha using lentiviral vectors. Long-term transduced skeletal progenitors were characterized by an enhanced production of cAMP, indicating the transfer of the fundamental cellular phenotype caused by activating mutations of Gs alpha. Like skeletal progenitors isolated from natural fibrous dysplasia (FD) lesions, transduced cells could generate bone but not adipocytes or the hematopoietic microenvironment on in vivo transplantation. In vitro osteogenic differentiation was noted for the lack of mineral deposition, a blunted upregulation of osteocalcin, and enhanced upregulation of other osteogenic markers such as alkaline phosphatase (ALP) and bone sialoprotein (BSP) compared with controls. A very potent upregulation of RANKL expression was observed, which correlates with the pronounced osteoclastogenesis observed in FD lesions in vivo. Stable transduction resulted in a marked upregulation of selected phosphodiesterase (PDE) isoform mRNAs and a prominent increase in total PDE activity. This predicts an adaptive response in skeletal progenitors transduced with constitutively active, mutated Gs alpha. Indeed, like measurable cAMP levels, the differentiative responses of transduced skeletal progenitors were profoundly affected by inhibition of PDEs or lack thereof. Finally, using lentiviral vectors encoding short hairpin (sh) RNA interfering sequences, we demonstrated that selective silencing of the mutated allele is both feasible and effective in reverting the aberrant cAMP production brought about by the constitutively active Gs alpha and some of its effects on in vitro differentiation of skeletal progenitors.
(c) 2010 American Society for Bone and Mineral Research.
Figures




References
-
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune‐Albright syndrome. N Engl J Med 1991; 325: 1688–1695. - PubMed
-
- Shenker A, Weinstein LS, Moran A, et al. Severe endocrine and nonendocrine manifestations of the McCune‐Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr 1993; 123: 509–518. - PubMed
-
- Masters SB, Miller RT, Chi MH, et al. Mutations in the GTP‐binding site of GSα alter stimulation of adenylyl cyclase. J Biol Chem 1989; 264: 15467–15474. - PubMed
-
- Bourne HR, Landis CA, Masters SB. Hydrolysis of GTP by the α‐chain of Gs and other GTP‐binding proteins. Proteins 1989; 6: 222–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

