Ability of VKORC1 and CYP2C9 to predict therapeutic warfarin dose during the initial weeks of therapy
- PMID: 19874474
- PMCID: PMC3718044
- DOI: 10.1111/j.1538-7836.2009.03677.x
Ability of VKORC1 and CYP2C9 to predict therapeutic warfarin dose during the initial weeks of therapy
Abstract
Background: CYP2C9 and VKORC1 genotypes predict therapeutic warfarin dose at initiation of therapy; however, the predictive ability of genetic information after a week or longer is unknown. Experts have hypothesized that genotype becomes irrelevant once international normalized ratio (INR) values are available because INR response reflects warfarin sensitivity.
Methods: We genotyped the participants in the Prevention of Recurrent Venous Thromboembolism (PREVENT) trial, who had idiopathic venous thromboemboli and began low-intensity warfarin (therapeutic INR 1.5-2.0) using a standard dosing protocol. To develop pharmacogenetic models, we quantified the effect of genotypes, clinical factors, previous doses and INR on therapeutic warfarin dose in the 223 PREVENT participants who were randomized to warfarin and achieved stable therapeutic INRs.
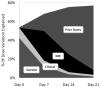
Results: A pharmacogenetic model using data from day 0 (before therapy initiation) explained 54% of the variability in therapeutic dose (R(2)). The R(2) increased to 68% at day 7, 75% at day 14, and 77% at day 21, because of increasing contributions from prior doses and INR response. Although CYP2C9 and VKORC1 genotypes were significant independent predictors of therapeutic dose at each weekly interval, the magnitude of their predictive ability diminished over time: partial R(2) of genotype was 43% at day 0, 12% at day 7, 4% at day 14, and 1% at day 21.
Conclusion: Over the first weeks of warfarin therapy, INR and prior dose become increasingly predictive of therapeutic dose, and genotype becomes less relevant. However, at day 7, genotype remains clinically relevant, accounting for 12% of therapeutic dose variability.
Figures
References
-
- Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, Rettie AE. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–8. - PubMed
-
- Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–93. - PubMed
-
- Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, Lu MJ, Hung CR, Wei CY, Chen CH, Wu JY, Chen YT. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14:1745–51. - PubMed
-
- Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, Milligan PE, Grice G, Lenzini P, Rettie AE, Aquilante CL, Grosso L, Marsh S, Langaee T, Farnett LE, Voora D, Veenstra DL, Glynn RJ, Barrett A, McLeod HL. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clinical pharmacology and therapeutics. 2008;84:326–31. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases