PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis
- PMID: 19874541
- PMCID: PMC2807913
- DOI: 10.1111/j.1365-313X.2009.04054.x
PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis
Abstract
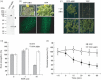
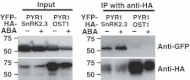
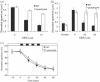
Abscisic acid (ABA) mediates resistance to abiotic stress and controls developmental processes in plants. The group-A PP2Cs, of which ABI1 is the prototypical member, are protein phosphatases that play critical roles as negative regulators very early in ABA signal transduction. Because redundancy is thought to limit the genetic dissection of early ABA signalling, to identify redundant and early ABA signalling proteins, we pursued a proteomics approach. We generated YFP-tagged ABI1 Arabidopsis expression lines and identified in vivo ABI1-interacting proteins by mass-spectrometric analyses of ABI1 complexes. Known ABA signalling components were isolated including SnRK2 protein kinases. We confirm previous studies in yeast and now show that ABI1 interacts with the ABA-signalling kinases OST1, SnRK2.2 and SnRK2.3 in plants. Interestingly, the most robust in planta ABI1-interacting proteins in all LC-MS/MS experiments were nine of the 14 PYR/PYL/RCAR proteins, which were recently reported as ABA-binding signal transduction proteins, providing evidence for in vivo PYR/PYL/RCAR interactions with ABI1 in Arabidopsis. ABI1-PYR1 interaction was stimulated within 5 min of ABA treatment in Arabidopsis. Interestingly, in contrast, PYR1 and SnRK2.3 co-immunoprecipitated equally well in the presence and absence of ABA. To investigate the biological relevance of the PYR/PYLs, we analysed pyr1/pyl1/pyl2/pyl4 quadruple mutant plants and found strong insensitivities in ABA-induced stomatal closure and ABA-inhibition of stomatal opening. These findings demonstrate that ABI1 can interact with several PYR/PYL/RCAR family members in Arabidopsis, that PYR1-ABI1 interaction is rapidly stimulated by ABA in Arabidopsis and indicate new SnRK2 kinase-PYR/PYL/RCAR interactions in an emerging model for PYR/PYL/RCAR-mediated ABA signalling.
Figures


References
-
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053–1067. - PubMed
-
- Batistic O, Kudla J. Plant calcineurin B-like proteins and their interacting protein kinases. Biochim. Biophys. Acta. 2009;1793:985–992. - PubMed
-
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

