Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells
- PMID: 19874627
- PMCID: PMC2780994
- DOI: 10.1186/1465-9921-10-102
Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells
Abstract
Background: Highly pathogenic avian influenza (HPAI) H5N1 virus is entrenched in poultry in Asia and Africa and continues to infect humans zoonotically causing acute respiratory disease syndrome and death. There is evidence that the virus may sometimes spread beyond respiratory tract to cause disseminated infection. The primary target cell for HPAI H5N1 virus in human lung is the alveolar epithelial cell. Alveolar epithelium and its adjacent lung microvascular endothelium form host barriers to the initiation of infection and dissemination of influenza H5N1 infection in humans. These are polarized cells and the polarity of influenza virus entry and egress as well as the secretion of cytokines and chemokines from the virus infected cells are likely to be central to the pathogenesis of human H5N1 disease.
Aim: To study influenza A (H5N1) virus replication and host innate immune responses in polarized primary human alveolar epithelial cells and lung microvascular endothelial cells and its relevance to the pathogenesis of human H5N1 disease.
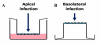
Methods: We use an in vitro model of polarized primary human alveolar epithelial cells and lung microvascular endothelial cells grown in transwell culture inserts to compare infection with influenza A subtype H1N1 and H5N1 viruses via the apical or basolateral surfaces.
Results: We demonstrate that both influenza H1N1 and H5N1 viruses efficiently infect alveolar epithelial cells from both apical and basolateral surface of the epithelium but release of newly formed virus is mainly from the apical side of the epithelium. In contrast, influenza H5N1 virus, but not H1N1 virus, efficiently infected polarized microvascular endothelial cells from both apical and basolateral aspects. This provides a mechanistic explanation for how H5N1 virus may infect the lung from systemic circulation. Epidemiological evidence has implicated ingestion of virus-contaminated foods as the source of infection in some instances and our data suggests that viremia, secondary to, for example, gastro-intestinal infection, can potentially lead to infection of the lung. HPAI H5N1 virus was a more potent inducer of cytokines (e.g. IP-10, RANTES, IL-6) in comparison to H1N1 virus in alveolar epithelial cells, and these virus-induced chemokines were secreted onto both the apical and basolateral aspects of the polarized alveolar epithelium.
Conclusion: The predilection of viruses for different routes of entry and egress from the infected cell is important in understanding the pathogenesis of influenza H5N1 infection and may help unravel the pathogenesis of human H5N1 disease.
Figures
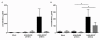
References
-
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. http://www.who.int/csr/disease/avian_influenza/country/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

