Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury
- PMID: 19874785
- PMCID: PMC2773653
- DOI: 10.1016/j.neuron.2009.09.016
Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury
Abstract
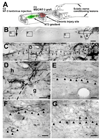
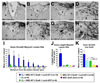
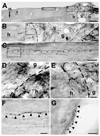
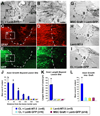
Despite advances in promoting axonal regeneration after acute spinal cord injury (SCI), elicitation of bridging axon regeneration after chronic SCI remains a formidable challenge. We report that combinatorial therapies administered 6 weeks, and as long as 15 months, after SCI promote axonal regeneration into and beyond a midcervical lesion site. Provision of peripheral nerve conditioning lesions, grafts of marrow stromal cells, and establishment of NT-3 gradients supports bridging regeneration. Controls receiving partial components of the full combination fail to exhibit bridging. Notably, intraneuronal molecular mechanisms recruited by delayed therapies mirror those of acute injury, including activation of transcriptional activators and regeneration-associated genes. Collectively, these findings provide evidence that regeneration is achievable at unprecedented postinjury time points.
Figures
References
-
- Bunge MB. Bridging areas of injury in the spinal cord. Neuroscientist. 2001;7:325–339. - PubMed
-
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. - PubMed
-
- Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

