Characterization of a carbon-carbon hydrolase from Mycobacterium tuberculosis involved in cholesterol metabolism
- PMID: 19875455
- PMCID: PMC2804191
- DOI: 10.1074/jbc.M109.058081
Characterization of a carbon-carbon hydrolase from Mycobacterium tuberculosis involved in cholesterol metabolism
Abstract
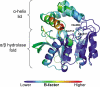
In the recently identified cholesterol catabolic pathway of Mycobacterium tuberculosis, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase (HsaD) is proposed to catalyze the hydrolysis of a carbon-carbon bond in 4,5-9,10-diseco-3-hydroxy-5,9,17-tri-oxoandrosta-1(10),2-diene-4-oic acid (DSHA), the cholesterol meta-cleavage product (MCP) and has been implicated in the intracellular survival of the pathogen. Herein, purified HsaD demonstrated 4-33 times higher specificity for DSHA (k(cat)/K(m) = 3.3 +/- 0.3 x 10(4) m(-1) s(-1)) than for the biphenyl MCP 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid (HOPDA) and the synthetic analogue 8-(2-chlorophenyl)-2-hydroxy-5-methyl-6-oxoocta-2,4-dienoic acid (HOPODA), respectively. The S114A variant of HsaD, in which the active site serine was substituted with alanine, was catalytically impaired and bound DSHA with a K(d) of 51 +/- 2 mum. The S114A.DSHA species absorbed maximally at 456 nm, 60 nm red-shifted versus the DSHA enolate. Crystal structures of the variant in complex with HOPDA, HOPODA, or DSHA to 1.8-1.9 Aindicate that this shift is due to the enzyme-induced strain of the enolate. These data indicate that the catalytic serine catalyzes tautomerization. A second role for this residue is suggested by a solvent molecule whose position in all structures is consistent with its activation by the serine for the nucleophilic attack of the substrate. Finally, the alpha-helical lid covering the active site displayed a ligand-dependent conformational change involving differences in side chain carbon positions of up to 6.7 A, supporting a two-conformation enzymatic mechanism. Overall, these results provide novel insights into the determinants of specificity in a mycobacterial cholesterol-degrading enzyme as well as into the mechanism of MCP hydrolases.
Figures
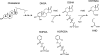






References
-
- Dye C. (2006) Lancet 367, 938–940 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

