The phosphatidylinositol 3-kinase/Akt pathway regulates transforming growth factor-{beta} signaling by destabilizing ski and inducing Smad7
- PMID: 19875456
- PMCID: PMC2790973
- DOI: 10.1074/jbc.M109.029488
The phosphatidylinositol 3-kinase/Akt pathway regulates transforming growth factor-{beta} signaling by destabilizing ski and inducing Smad7
Abstract
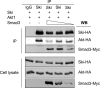
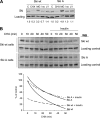
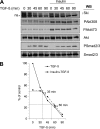
Ski is an oncoprotein that negatively regulates transforming growth factor (TGF)-beta signaling. It acts as a transcriptional co-repressor by binding to TGF-beta signaling molecules, Smads. Efficient TGF-beta signaling is facilitated by rapid proteasome-mediated degradation of Ski by TGF-beta. Here we report that Ski is phosphorylated by Akt/PKB kinase. Akt phosphorylates Ski on a highly conserved Akt motif at threonine 458 both in vitro and in vivo. The phosphorylation of Ski at threonine 458 is induced by Akt pathway activators including insulin, insulin-like growth factor-1, and hepatocyte growth factor. The phosphorylation of Ski causes its destabilization and reduces Ski-mediated inhibition of expression of another negative regulator of TGF-beta, Smad7. Induction of Smad7 levels leads to inactivation of TGF-beta receptors and TGF-beta signaling cascade, as indicated by reduced induction of TGF-beta target p15. Therefore, Akt modulates TGF-beta signaling by temporarily adjusting the levels of two TGF-beta pathway negative regulators, Ski and Smad7. These novel findings demonstrate that Akt pathway activation directly impacts TGF-beta pathway.
Figures





References
-
- Massagué J. (1998) Annu. Rev. Biochem. 67, 753–791 - PubMed
-
- Lönn P., Morén A., Raja E., Dahl M., Moustakas A. (2009) Cell Res. 19, 21–35 - PubMed
-
- Itóh S., ten Dijke P. (2007) Curr. Opin. Cell Biol. 19, 176–184 - PubMed
-
- Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J. L., Heuchel R., Itoh S., Kawabata M., Heldin N. E., Heldin C. H., ten Dijke P. (1997) Nature 389, 631–635 - PubMed
-
- Hayashi H., Abdollah S., Qiu Y., Cai J., Xu Y. Y., Grinnell B. W., Richardson M. A., Topper J. N., Gimbrone M. A., Jr., Wrana J. L., Falb D. (1997) Cell 89, 1165–1173 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

