Hyperpolarized MR imaging: neurologic applications of hyperpolarized metabolism
- PMID: 19875468
- PMCID: PMC7964072
- DOI: 10.3174/ajnr.A1790
Hyperpolarized MR imaging: neurologic applications of hyperpolarized metabolism
Abstract
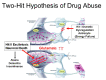
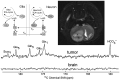
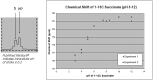
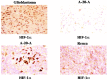
Hyperpolarization is the general term for a method of enhancing the spin-polarization difference of populations of nuclei in a magnetic field. No less than 5 distinct techniques (dynamic nuclear polarization [DNP]; parahydrogen-induced polarization-parahydrogen and synthesis allow dramatically enhanced nuclear alignment [PHIP-PASADENA]; xenon/helium polarization transfer; Brute Force; (1)H hyperpolarized water) are currently under exhaustive investigation as means of amplifying the intrinsically (a few parts per million) weak signal intensity used in conventional MR neuroimaging and spectroscopy. HD-MR imaging in vivo is a metabolic imaging tool causing much of the interest in HD-MR imaging. The most successful to date has been DNP, in which carbon-13 ((13)C) pyruvic acid has shown many. PHIP-PASADENA with (13)C succinate has shown HD-MR metabolism in vivo in tumor-bearing mice of several types, entering the Krebs-tricarboxylic acid cycle for ultrafast detection with (13)C MR imaging, MR spectroscopy, and chemical shift imaging. We will discuss 5 promising preclinical studies: (13)C succinate PHIP in brain tumor; (13)C ethylpyruvate DNP and (13)C acetate; DNP in rodent brain; (13)C succinate PHIP versus gadolinium imaging of stroke; and (1)H hyperpolarized imaging. Recent developments in clinical (13)C neurospectroscopy encourage us to overcome the remaining barriers to clinical HD-MR imaging.
Figures









References
-
- Barker P. How to enhance your image. Advanced Imaging Seminar: Contrast Agents. Proc ASNR; 2009, Vancouver, Canada.
-
- Ross B, Lin A, Harris K, et al. . Clinical experience with 13C MRS in vivo. NMR Biomed 2003; 16: 358–69 - PubMed
-
- Sailasuta N, Harris KC, Abulseoud O, et al. . 1-13C acetate MRS to study glial glutamate dysfunction in methamphetamine users. Proc Int Soc Magn Reson Med 2009; 17: 92
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
