Metabolomics applied to diabetes research: moving from information to knowledge
- PMID: 19875619
- PMCID: PMC2768174
- DOI: 10.2337/db09-0580
Metabolomics applied to diabetes research: moving from information to knowledge
Figures
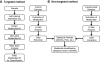

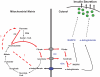



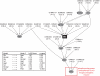
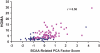
References
-
- Ridderstråle M, Groop L: Genetic dissection of type 2 diabetes. Mol Cell Endocrinol 2009;297:10–17 - PubMed
-
- Muoio DM, Newgard CB: Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 - PubMed
-
- Human Metabolome Project: Human Metabolome Database-Canada [website]. Available at http://www.hmdb.ca/ Accessed 19 September 2009
-
- Castle AL, Fiehn O, Kaddurah-Daouk R, Lindon JC: Metabolomics Standards Workshop and the development of international standards for reporting metabolomics experimental results. Brief Bioinform 2006;7:159–165 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

