Human corneal fibrosis: an in vitro model
- PMID: 19875671
- PMCID: PMC2868432
- DOI: 10.1167/iovs.09-3860
Human corneal fibrosis: an in vitro model
Abstract
Purpose: Corneal injury may ultimately lead to a scar by way of corneal fibrosis, which is characterized by the presence of myofibroblasts and improper deposition of extracellular matrix (ECM) components. TGF-beta1 is known to stimulate overproduction and deposition of ECM components. Previously, an in vitro three-dimensional (3-D) model of a corneal stroma was developed by using primary human corneal fibroblasts (HCFs) stimulated with stable vitamin C (VitC). This model mimics corneal development. The authors postulate that with the addition of TGF-beta1, a 3-D corneal scar model can be generated.
Methods: HCFs were grown in four media conditions for 4 or 8 weeks: VitC only; VitC+TGF-beta1 for the entire time; VitC+TGF-beta1 for 1 week, then VitC only for 3 or 7 weeks; and VitC for 4 weeks, then VitC+TGF-beta1 for 4 weeks. Cultures were analyzed with TEM and indirect immunofluorescence.
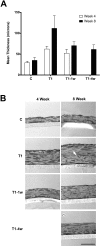
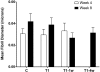
Results: Compared with the control, addition of TGF-beta1 increased construct thickness significantly, with maximum increase in constructs with TGF-beta1 present for the entire time-2.1- to 3.2-fold at 4 and 8 weeks, respectively. In all TGF-beta-treated cultures, cells became long and flat, numerous filamentous cells were seen, collagen levels increased, and long collagen fibrils were visible. Smooth muscle actin, cellular fibronectin, and type III collagen expression all appeared to increase. Cultures between weeks 4 and 8 showed minimal differences.
Conclusions: Human corneal fibroblasts stimulated by VitC and TGF-beta1 appear to generate a model that resembles processes observed in human corneal fibrosis. This model should be useful in examining matrix deposition and assembly in a wound-healing situation.
Figures




References
-
- Pouliquen YJ. 1984 Castroviejo lecture: fine structure of the corneal stroma. Cornea 1984;3:168–177 - PubMed
-
- Hamada R, Giraud JP, Graf B, Pouliquen Y. Analytical and statistical study of the lamellae, keratocytes and collagen fibrils of the central region of the normal human cornea (light and electron microscopy) (in French). Arch Ophtalmol Rev Gen Ophtalmol 1972;32:563–570 - PubMed
-
- Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res 2001;72:33–39 - PubMed
-
- Hata R, Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol 1989;138:8–16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

