Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates
- PMID: 19875694
- PMCID: PMC2770441
- DOI: 10.1073/pnas.0907655106
Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates
Abstract
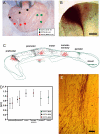
The corpus callosum (CC) provides the main route of communication between the 2 hemispheres of the brain. In monkeys, chimpanzees, and humans, callosal axons of distinct size interconnect functionally different cortical areas. Thinner axons in the genu and in the posterior body of the CC interconnect the prefrontal and parietal areas, respectively, and thicker axons in the midbody and in the splenium interconnect primary motor, somatosensory, and visual areas. At all locations, axon diameter, and hence its conduction velocity, increases slightly in the chimpanzee compared with the macaque because of an increased number of large axons but not between the chimpanzee and man. This, together with the longer connections in larger brains, doubles the expected conduction delays between the hemispheres, from macaque to man, and amplifies their range about 3-fold. These changes can have several consequences for cortical dynamics, particularly on the cycle of interhemispheric oscillators.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: A conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. - PubMed
-
- Olivares R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behav Evol. 2001;57:98–105. - PubMed
-
- Innocenti GM, Aggoun-Zouaoui D, Lehmann P. Cellular aspects of callosal connections and their development. Neuropsychologia. 1995;33:961–987. - PubMed
-
- LaMantia A-S, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol. 1990;291:520–537. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

