Renal dendritic cells ameliorate nephrotoxic acute kidney injury
- PMID: 19875815
- PMCID: PMC2799272
- DOI: 10.1681/ASN.2009040407
Renal dendritic cells ameliorate nephrotoxic acute kidney injury
Abstract
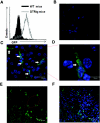
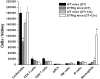
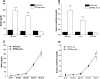
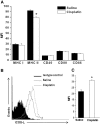
Inflammation contributes to the pathogenesis of acute kidney injury. Dendritic cells (DCs) are immune sentinels with the ability to induce immunity or tolerance, but whether they mediate acute kidney injury is unknown. Here, we studied the distribution of DCs within the kidney and the role of DCs in cisplatin-induced acute kidney injury using a mouse model in which DCs express both green fluorescence protein and the diphtheria toxin receptor. DCs were present throughout the tubulointerstitium but not in glomeruli. We used diphtheria toxin to deplete DCs to study their functional significance in cisplatin nephrotoxicity. Mice depleted of DCs before or coincident with cisplatin treatment but not at later stages experienced more severe renal dysfunction, tubular injury, neutrophil infiltration and greater mortality than nondepleted mice. We used bone marrow chimeric mice to confirm that the depletion of CD11c-expressing hematopoietic cells was responsible for the enhanced renal injury. Finally, mixed bone marrow chimeras demonstrated that the worsening of cisplatin nephrotoxicity in DC-depleted mice was not a result of the dying or dead DCs themselves. After cisplatin treatment, expression of MHC class II decreased and expression of inducible co-stimulator ligand increased on renal DCs. These data demonstrate that resident DCs reduce cisplatin nephrotoxicity and its associated inflammation.
Figures

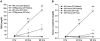


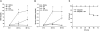


References
-
- Ramesh G, Reeves WB: TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285: 610–618, 2003 - PubMed
-
- Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 - PubMed
-
- Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007 - PubMed
-
- Zhang B, Ramesh G, Norbury CC, Reeves WB: Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int 72: 37–44, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

