Specific entrainment of mitral cells during gamma oscillation in the rat olfactory bulb
- PMID: 19876377
- PMCID: PMC2760751
- DOI: 10.1371/journal.pcbi.1000551
Specific entrainment of mitral cells during gamma oscillation in the rat olfactory bulb
Abstract
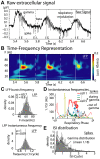
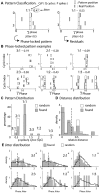
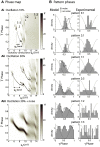
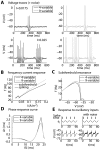
Local field potential (LFP) oscillations are often accompanied by synchronization of activity within a widespread cerebral area. Thus, the LFP and neuronal coherence appear to be the result of a common mechanism that underlies neuronal assembly formation. We used the olfactory bulb as a model to investigate: (1) the extent to which unitary dynamics and LFP oscillations can be correlated and (2) the precision with which a model of the hypothesized underlying mechanisms can accurately explain the experimental data. For this purpose, we analyzed simultaneous recordings of mitral cell (MC) activity and LFPs in anesthetized and freely breathing rats in response to odorant stimulation. Spike trains were found to be phase-locked to the gamma oscillation at specific firing rates and to form odor-specific temporal patterns. The use of a conductance-based MC model driven by an approximately balanced excitatory-inhibitory input conductance and a relatively small inhibitory conductance that oscillated at the gamma frequency allowed us to provide one explanation of the experimental data via a mode-locking mechanism. This work sheds light on the way network and intrinsic MC properties participate in the locking of MCs to the gamma oscillation in a realistic physiological context and may result in a particular time-locked assembly. Finally, we discuss how a self-synchronization process with such entrainment properties can explain, under experimental conditions: (1) why the gamma bursts emerge transiently with a maximal amplitude position relative to the stimulus time course; (2) why the oscillations are prominent at a specific gamma frequency; and (3) why the oscillation amplitude depends on specific stimulus properties. We also discuss information processing and functional consequences derived from this mechanism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Respiration-gated formation of gamma and beta neural assemblies in the mammalian olfactory bulb.Eur J Neurosci. 2009 Mar;29(5):921-30. doi: 10.1111/j.1460-9568.2009.06651.x. Eur J Neurosci. 2009. PMID: 19291223
-
Odorant modulation of neuronal activity and local field potential in sensory-deprived olfactory bulb.Neuroscience. 2009 Sep 15;162(4):1265-78. doi: 10.1016/j.neuroscience.2009.05.051. Epub 2009 May 27. Neuroscience. 2009. PMID: 19481588
-
Two distinct olfactory bulb sublaminar networks involved in gamma and beta oscillation generation: a CSD study in the anesthetized rat.Front Neural Circuits. 2014 Jul 30;8:88. doi: 10.3389/fncir.2014.00088. eCollection 2014. Front Neural Circuits. 2014. PMID: 25126057 Free PMC article.
-
Oscillations and Spike Entrainment.F1000Res. 2018 Dec 20;7:F1000 Faculty Rev-1960. doi: 10.12688/f1000research.16451.1. eCollection 2018. F1000Res. 2018. PMID: 30613382 Free PMC article. Review.
-
Circuit oscillations in odor perception and memory.Prog Brain Res. 2014;208:223-51. doi: 10.1016/B978-0-444-63350-7.00009-7. Prog Brain Res. 2014. PMID: 24767485 Review.
Cited by
-
Precise detection of direct glomerular input duration by the olfactory bulb.J Neurosci. 2014 Nov 26;34(48):16058-64. doi: 10.1523/JNEUROSCI.3382-14.2014. J Neurosci. 2014. PMID: 25429146 Free PMC article.
-
Neurophysiological and computational principles of cortical rhythms in cognition.Physiol Rev. 2010 Jul;90(3):1195-268. doi: 10.1152/physrev.00035.2008. Physiol Rev. 2010. PMID: 20664082 Free PMC article. Review.
-
Respiration drives network activity and modulates synaptic and circuit processing of lateral inhibition in the olfactory bulb.J Neurosci. 2012 Jan 4;32(1):85-98. doi: 10.1523/JNEUROSCI.4278-11.2012. J Neurosci. 2012. PMID: 22219272 Free PMC article.
-
Encoding odorant identity by spiking packets of rate-invariant neurons in awake mice.PLoS One. 2012;7(1):e30155. doi: 10.1371/journal.pone.0030155. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272291 Free PMC article.
-
A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task.J Neurophysiol. 2010 Aug;104(2):829-39. doi: 10.1152/jn.00166.2010. Epub 2010 Jun 10. J Neurophysiol. 2010. PMID: 20538778 Free PMC article.
References
-
- Cassenaer S, Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature. 2007;448:709–713. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

