Mycobacteria exploit host hyaluronan for efficient extracellular replication
- PMID: 19876387
- PMCID: PMC2763203
- DOI: 10.1371/journal.ppat.1000643
Mycobacteria exploit host hyaluronan for efficient extracellular replication
Abstract
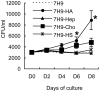
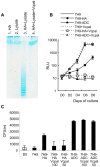
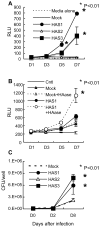
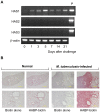
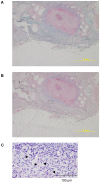
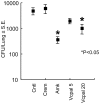
In spite of the importance of hyaluronan in host protection against infectious organisms in the alveolar spaces, its role in mycobacterial infection is unknown. In a previous study, we found that mycobacteria interact with hyaluronan on lung epithelial cells. Here, we have analyzed the role of hyaluronan after mycobacterial infection was established and found that pathogenic mycobacteria can grow by utilizing hyaluronan as a carbon source. Both mouse and human possess 3 kinds of hyaluronan synthases (HAS), designated HAS1, HAS2, and HAS3. Utilizing individual HAS-transfected cells, we show that HAS1 and HAS3 but not HAS2 support growth of mycobacteria. We found that the major hyaluronan synthase expressed in the lung is HAS1, and that its expression was increased after infection with Mycobacterium tuberculosis. Histochemical analysis demonstrated that hyaluronan profoundly accumulated in the granulomatous legion of the lungs in M. tuberculosis-infected mice and rhesus monkeys that died from tuberculosis. We detected hyaluronidase activity in the lysate of mycobacteria and showed that it was critical for hyaluronan-dependent extracellular growth. Finally, we showed that L-Ascorbic acid 6-hexadecanoate, a hyaluronidase inhibitor, suppressed growth of mycobacteria in vivo. Taken together, our data show that pathogenic mycobacteria exploit an intrinsic host-protective molecule, hyaluronan, to grow in the respiratory tract and demonstrate the potential usefulness of hyaluronidase inhibitors against mycobacterial diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Aoki K, Matsumoto S, Hirayama Y, Wada T, Ozeki Y, et al. Extracellular mycobacterial DNA-binding protein 1 participates in Mycobacterium-lung epithelial cell interaction through hyaluronic acid. J Biol Chem. 2004;279:39798–39806. - PubMed
-
- Hernandez-Pando R, Jeyanathan M, Mengistu G, Aguilar D, Orozco H, et al. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–2138. - PubMed
-
- Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, et al. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10:641–650. - PubMed
-
- Nishimura J, Saiga H, Sato S, Okuyama M, Kayama H, et al. Potent antimycobacterial activity of mouse secretory leukocyte protease inhibitor. J Immunol. 2008;180:4032–4039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

