Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans
- PMID: 19876394
- PMCID: PMC2763347
- DOI: 10.1371/journal.ppat.1000639
Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans
Abstract
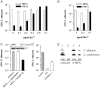
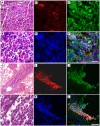
Neutrophils are the first line of defense at the site of an infection. They encounter and kill microbes intracellularly upon phagocytosis or extracellularly by degranulation of antimicrobial proteins and the release of Neutrophil Extracellular Traps (NETs). NETs were shown to ensnare and kill microbes. However, their complete protein composition and the antimicrobial mechanism are not well understood. Using a proteomic approach, we identified 24 NET-associated proteins. Quantitative analysis of these proteins and high resolution electron microscopy showed that NETs consist of modified nucleosomes and a stringent selection of other proteins. In contrast to previous results, we found several NET proteins that are cytoplasmic in unstimulated neutrophils. We demonstrated that of those proteins, the antimicrobial heterodimer calprotectin is released in NETs as the major antifungal component. Absence of calprotectin in NETs resulted in complete loss of antifungal activity in vitro. Analysis of three different Candida albicans in vivo infection models indicated that NET formation is a hitherto unrecognized route of calprotectin release. By comparing wild-type and calprotectin-deficient animals we found that calprotectin is crucial for the clearance of infection. Taken together, the present investigations confirmed the antifungal activity of calprotectin in vitro and, moreover, demonstrated that it contributes to effective host defense against C. albicans in vivo. We showed for the first time that a proportion of calprotectin is bound to NETs in vitro and in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Geiszt M, Kapus A, Ligeti E. Chronic granulomatous disease: more than the lack of superoxide? J Leukoc Biol. 2001;69:191–196. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. - PubMed
-
- von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. - PubMed
-
- Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

