The placenta in the integrated physiology of fetal volume control
- PMID: 19876827
- PMCID: PMC3402164
- DOI: 10.1387/ijdb.082796jf
The placenta in the integrated physiology of fetal volume control
Abstract
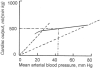
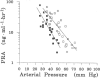
Almost all water that enters the conceptus of the sheep enters via the placenta. The forces that drive water are hydrostatic and osmotic. The placental channels that allow water to cross into the fetus have not been identified by microanatomic means. Although an "equivalent pore" system can account for the diffusional entry of small hydrophilic solutes, it can be calculated that the filtration coefficient of this system is too small to account for the demonstrated trans-placental water flows. It is possible that a second much less numerous system of large pores permits the flow of water, but that is by no means certain. The placenta does not control the amount of water that enters the conceptus; nor does any other single fetal structure. And water entry is not dependent on the volume of water already present. However, the combined physiological properties of the fetal heart, kidneys, somatic tissues and placenta constitute a consistent explanation of fetal water volume control.
Figures

Similar articles
-
Current topic: water volume of the ovine conceptus; point of view.Placenta. 1992 May-Jun;13(3):199-212. doi: 10.1016/0143-4004(92)90035-r. Placenta. 1992. PMID: 1635908 Review.
-
Filtration of water from mother to conceptus via paths independent of fetal placental circulation in sheep.J Physiol. 1990 Dec;431:1-10. doi: 10.1113/jphysiol.1990.sp018317. J Physiol. 1990. PMID: 2100302 Free PMC article.
-
Maternal-fetal fluid balance and aquaporins: from molecule to physiology.Acta Pharmacol Sin. 2011 Jun;32(6):716-20. doi: 10.1038/aps.2011.59. Epub 2011 May 23. Acta Pharmacol Sin. 2011. PMID: 21602839 Free PMC article. Review.
-
The dynamic placenta: II. Hypothetical model of a fetus driven transplacental water balance mechanism producing low apparent permeability in a highly permeable placenta.Med Hypotheses. 2004;62(4):520-8. doi: 10.1016/j.mehy.2003.10.019. Med Hypotheses. 2004. PMID: 15050099
-
Model study of placental water transfer and causes of fetal water disease in sheep.Am J Physiol. 1990 May;258(5 Pt 2):R1257-70. doi: 10.1152/ajpregu.1990.258.5.R1257. Am J Physiol. 1990. PMID: 2186642
Cited by
-
Ovine uterine space restriction causes dysregulation of the renin-angiotensin system in fetal kidneys.Biol Reprod. 2017 Jan 1;96(1):211-220. doi: 10.1095/biolreprod.116.140079. Biol Reprod. 2017. PMID: 28395333 Free PMC article.
-
The kidney transcriptome and proteome defined by transcriptomics and antibody-based profiling.PLoS One. 2014 Dec 31;9(12):e116125. doi: 10.1371/journal.pone.0116125. eCollection 2014. PLoS One. 2014. PMID: 25551756 Free PMC article.
-
Insignificant response of the fetal placental circulation to arterial hypotension in sheep.J Appl Physiol (1985). 2011 Oct;111(4):1042-7. doi: 10.1152/japplphysiol.00345.2011. Epub 2011 Jun 30. J Appl Physiol (1985). 2011. PMID: 21719727 Free PMC article.
-
Arterio-venous fetoplacental vascular geometry and hemodynamics in the mouse placenta.Placenta. 2017 Oct;58:46-51. doi: 10.1016/j.placenta.2017.08.007. Epub 2017 Aug 12. Placenta. 2017. PMID: 28962695 Free PMC article.
-
An observational study of associations among maternal fluids during parturition, neonatal output, and breastfed newborn weight loss.Int Breastfeed J. 2011 Aug 15;6:9. doi: 10.1186/1746-4358-6-9. Int Breastfeed J. 2011. PMID: 21843338 Free PMC article.
References
-
- ADAMSON SL, MORROW RJ, BULL SB, LANGILLE BL. Vasomotor responses of the umbilical circulation in fetal sheep. Am J Physiol. 1989;256:R1056–R1062. - PubMed
-
- ADAMSON SL, WHITELEY KJ, LANGILLE BL. Pulsatile pressureflow relations and pulse wave propagation in the umbilical circulation of fetal sheep. Circ Res. 1992;70:761–772. - PubMed
-
- ANDERSON DF, BORST CG, FABER JJ. Excess extrafetal fluid without demonstrable changes in placental concentration gradients after weeklong infusions of angiotensin into fetal lambs. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995;63:175–179. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

