Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction
- PMID: 19876951
- PMCID: PMC2860694
- DOI: 10.1002/jbm.a.32632
Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction
Abstract
A bovine acellular scaffold was found to facilitate tissue remodeling in a rat model of vocal fold injury, whereas hepatocyte growth factor (HGF) has been shown to have an antiscarring effect in the larynx. This study examined the loading and release kinetics of HGF in vitro, and the potential of the acellular scaffold as a timed-release system for the delivery of HGF in vivo. Bilateral wounds were created in the posterior vocal folds of 20 rats, with HGF-loaded acellular scaffolds implanted into the wounds unilaterally, and scaffolds without HGF implanted into the contralateral vocal folds as control. The rats were humanely sacrificed after 3, 7, 30, and 90 days and their larynges were examined histologically and immunohistochemically. Expressions of key matrix proteins in the vocal fold coronal sections were quantified by digital image analysis. Results demonstrated a gradual, sustained release of HGF for at least 7 days in vitro, consistent with the detection of glycosaminoglycans inherent of the scaffold. In rat vocal folds implanted with HGF-loaded scaffolds, apparently fewer inflammatory cells were observed 3 days after surgery when compared to the control. The mean relative densities of collagen III and hyaluronic acid were significantly lower than those of the control 7 days after surgery. Scaffold implants were apparently degraded by 3 months in all animals, with no evidence of fibrosis or calcification. These data suggested that the bovine acellular scaffold could be promising for the exogenous delivery of select growth factors in vivo.
(c) 2009 Wiley Periodicals, Inc.
Figures


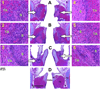
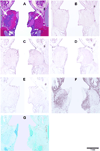

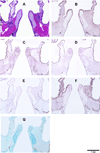


Similar articles
-
A bovine acellular scaffold for vocal fold reconstruction in a rat model.J Biomed Mater Res A. 2010 Jan;92(1):18-32. doi: 10.1002/jbm.a.32279. J Biomed Mater Res A. 2010. PMID: 19165789 Free PMC article.
-
Hepatocyte growth factor and its receptor c-met in rat and rabbit vocal folds.Ann Otol Rhinol Laryngol. 2002 Aug;111(8):661-6. doi: 10.1177/000348940211100801. Ann Otol Rhinol Laryngol. 2002. PMID: 12184584
-
Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts.Ann Otol Rhinol Laryngol. 2009 Aug;118(8):606-11. doi: 10.1177/000348940911800813. Ann Otol Rhinol Laryngol. 2009. PMID: 19746761
-
A narrative review of basic and clinical studies for vocal fold regeneration therapies.Auris Nasus Larynx. 2024 Dec;51(6):1052-1059. doi: 10.1016/j.anl.2024.10.011. Epub 2024 Oct 30. Auris Nasus Larynx. 2024. PMID: 39476786 Review.
-
Vocal fold injury models in rats: a literature review on techniques and methodology.J Med Life. 2022 Mar;15(3):336-343. doi: 10.25122/jml-2022-0032. J Med Life. 2022. PMID: 35449991 Free PMC article. Review.
Cited by
-
Paired versus two-group experimental design for rheological studies of vocal fold tissues.J Biomech. 2019 Jan 23;83:150-156. doi: 10.1016/j.jbiomech.2018.11.040. Epub 2018 Nov 29. J Biomech. 2019. PMID: 30579579 Free PMC article.
-
Nonlinear viscoelastic characterization of human vocal fold tissues under large-amplitude oscillatory shear (LAOS).J Rheol (N Y N Y). 2018 May;62(3):695-712. doi: 10.1122/1.4996320. Epub 2018 Apr 1. J Rheol (N Y N Y). 2018. PMID: 29780189 Free PMC article.
-
Dexamethasone Controlled Release on TGF-β1 Treated Vocal Fold Fibroblasts.Ann Otol Rhinol Laryngol. 2015 Jul;124(7):572-8. doi: 10.1177/0003489415570933. Epub 2015 Feb 9. Ann Otol Rhinol Laryngol. 2015. PMID: 25667215 Free PMC article.
-
Hyaluronic Acid/Alginate Hydrogel Containing Hepatocyte Growth Factor and Promotion of Vocal Fold Wound Healing.Tissue Eng Regen Med. 2020 Oct;17(5):651-658. doi: 10.1007/s13770-020-00280-6. Epub 2020 Jul 16. Tissue Eng Regen Med. 2020. PMID: 32676953 Free PMC article.
-
Implantation of atelocollagen sheet for vocal fold scar.Curr Opin Otolaryngol Head Neck Surg. 2010 Dec;18(6):507-11. doi: 10.1097/MOO.0b013e32833febdc. Curr Opin Otolaryngol Head Neck Surg. 2010. PMID: 20856118 Free PMC article. Review.
References
-
- Xu CC, Chan RW, Tirunagari N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007;13:551–566. - PubMed
-
- Xu CC, Chan RW. Pore architecture of a bovine acellular vocal fold scaffold. Tissue Eng Part A. 2008;14:1893–1903. - PubMed
-
- Pollard TD, Earnshaw WC. Cell biology. 1st ed. Philadelphia, PA: Elsevier Inc.; 2004. pp. 485–506.
-
- Campbell MK, Farrell SO. Biochemistry. 5th ed. Belmont, CA: Thomson Brooks/Cole; 2006. pp. 434–462.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

