Recrossing and dynamic matching effects on selectivity in a Diels-Alder reaction
- PMID: 19876990
- PMCID: PMC2810121
- DOI: 10.1002/anie.200903293
Recrossing and dynamic matching effects on selectivity in a Diels-Alder reaction
Abstract
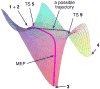
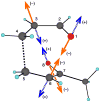
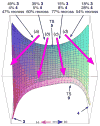
The products from the hetero-Diels-Alder reaction of acrolein with methyl vinyl ketone arise from a single transition state and trajectory studies accurately predict the selectivity. In an extension of the dynamic matching idea of Carpenter, the product formed is determined by the direction of motion passing through the transition state. Recrossing of the cycloaddition transition state occurs extensively and decreases formation of the minor product.
Figures

References
-
- Carpenter BK. Angew Chem. 1998;110:3532–3543.
- Angew Chem Int Ed. 1998;37:3340–3350. - PubMed
- Carpenter BK. J Phys Org Chem. 2003;16:858–868.
-
- Carpenter BK. J Am Chem Soc. 1985;107:5730–5732.
- Carpenter BK. J Am Chem Soc. 1995;117:6336–6344.
- Carpenter BK. J Am Chem Soc. 1996;118:10329–10330.
- Reyes MB, Carpenter BK. J Am Chem Soc. 2000;122:10163–10176.
- Reyes MB, Lobkovsky EB, Carpenter BK. J Am Chem Soc. 2002;124:641–651. - PubMed
- Nummela JA, Carpenter BK. J Am Chem Soc. 2002;124:8512–8513. - PubMed
- Litovitz AE, Keresztes I, Carpenter BK. J Am Chem Soc. 2008;130:12085–12094. - PMC - PubMed
-
- Doubleday C, Jr, Bolton K, Hase WL. J Am Chem Soc. 1997;119:5251–5252.
- Doubleday C, Nendel M, Houk KN, Thweatt D, Page M. J Am Chem Soc. 1999;121:4720–4721.
- Doubleday C, Suhrada CP, Houk KN. J Am Chem Soc. 2006;128:90–94. - PubMed
-
- Kless A, Nendel M, Wilsey S, Houk KN. J Am Chem Soc. 1999;121:4524–4525.
- Debbert SL, Carpenter BK, Hrovat DA, Borden WT. J Am Chem Soc. 2002;124:7896–7897. - PubMed
-
- Metiu H, Ross J, Silbey R, George TF. J Chem Phys. 1974;61:3200–3209.
- Valtazanos P, Ruedenberg K. Theor Chim Acta. 1986;69:281–307.
- Windus TL, Gordon MS, Burggraf LW, Davis LP. J Am Chem Soc. 1991;113:4356–4357.
- Tachibana A, Okazaki I, Koizumi M, Hori K, Yamabe T. J Am Chem Soc. 1985;107:1190–1196.
- Zhou C, Birney DM. Org Lett. 2002;4:3279–3282. - PubMed
- Wei H, Hrovat DA, Borden WT. J Am Chem Soc. 2006;128:16676–16683. - PubMed
- Shaik S, Danovich D, Sastry GN, Ayala PY, Schlegel HB. J Am Chem Soc. 1997;119:9237–9245.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

