A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner
- PMID: 19877052
- PMCID: PMC2783420
- DOI: 10.1002/art.24877
A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner
Abstract
Objective: Chronic T cell activation is central to the etiology of rheumatoid arthritis (RA), an inflammatory autoimmune disease that leads to severe focal bone erosions and generalized systemic osteoporosis. Previous studies have shown novel cytokine-like activities in medium containing activated T cells, characterized by potent induction of the osteoblastic production of interleukin-6 (IL-6), an inflammatory cytokine and stimulator of osteoclastogenesis, as well as induction of an activity that directly stimulates osteoclast formation in a manner independent of the key osteoclastogenic cytokine RANKL. This study was undertaken to identify the factors secreted by T cells that are responsible for these activities.
Methods: Human T cells were activated using anti-human CD3 and anti-human CD28 antibodies for 72 hours in AIM V serum-free medium to obtain T cell-conditioned medium, followed by concentration and fractionation of the medium by fast-protein liquid chromatography. Biologically active fractions were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Major bands were analyzed by mass spectrometry, and a major candidate protein was identified. This novel cytokine was cloned, and its expression was analyzed using recombinant DNA technologies.
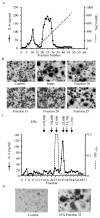
Results: A single novel cytokine that could induce both osteoblastic IL-6 production and functional osteoclast formation in the absence of osteoblasts or RANKL and that was insensitive to the effects of the RANKL inhibitor osteoprotegerin was identified in the activated T cell-conditioned medium; this cytokine was designated secreted osteoclastogenic factor of activated T cells (SOFAT). Further analysis of SOFAT revealed that it was derived from an unusual messenger RNA splice variant coded by the threonine synthase-like 2 gene homolog, which is a conserved gene remnant coding for threonine synthase, an enzyme that functions only in microorganisms and plants.
Conclusion: SOFAT may act to exacerbate inflammation and/or bone turnover under inflammatory conditions such as RA or periodontitis and in conditions of estrogen deficiency.
Figures





Comment in
-
Human osteoclastogenic T cells and human osteoclastology.Arthritis Rheum. 2009 Nov;60(11):3158-63. doi: 10.1002/art.24886. Arthritis Rheum. 2009. PMID: 19877050 No abstract available.
References
-
- Deodhar AA, Woolf AD. Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol. 1996;35(4):309–322. - PubMed
-
- Dequeker J, Maenaut K, Verwilghen J, Westhovens R. Osteoporosis in rheumatoid arthritis. Clin Exp Rheumatol. 1995;13 (Suppl 12):S21–6. - PubMed
-
- Fournier C. Where do T cells stand in rheumatoid arthritis? Joint Bone Spine. 2005;72(6):527–532. - PubMed
-
- Ogawa Y, Ohtsuki M, Uzuki M, Sawai T, Onozawa Y, Nakayama J, et al. Suppression of osteoclastogenesis in rheumatoid arthritis by induction of apoptosis in activated CD4+ T cells. Arthritis Rheum. 2003;48(12):3350–3358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

