Generation of functional human hepatic endoderm from human induced pluripotent stem cells
- PMID: 19877180
- PMCID: PMC2799548
- DOI: 10.1002/hep.23335
Generation of functional human hepatic endoderm from human induced pluripotent stem cells
Abstract
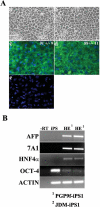
With the advent of induced pluripotent stem cell (iPSC) technology, it is now feasible to generate iPSCs with a defined genotype or disease state. When coupled with direct differentiation to a defined lineage, such as hepatic endoderm (HE), iPSCs would revolutionize the way we study human liver biology and generate efficient "off the shelf" models of human liver disease. Here, we show the "proof of concept" that iPSC lines representing both male and female sexes and two ethnic origins can be differentiated to HE at efficiencies of between 70%-90%, using a method mimicking physiological relevant condition. The iPSC-derived HE exhibited hepatic morphology and expressed the hepatic markers albumin and E-cadherin, as assessed by immunohistochemistry. They also expressed alpha-fetoprotein, hepatocyte nuclear factor-4a, and a metabolic marker, cytochrome P450 7A1 (Cyp7A1), demonstrating a definitive endodermal lineage differentiation. Furthermore, iPSC-derived hepatocytes produced and secreted the plasma proteins, fibrinogen, fibronectin, transthyretin, and alpha-fetoprotein, an essential feature for functional HE. Additionally iPSC-derived HE supported both CYP1A2 and CYP3A4 metabolism, which is essential for drug and toxicology testing.
Conclusion: This work is first to demonstrate the efficient generation of hepatic endodermal lineage from human iPSCs that exhibits key attributes of hepatocytes, and the potential application of iPSC-derived HE in studying human liver biology. In particular, iPSCs from individuals representing highly polymorphic variants in metabolic genes and different ethnic groups will provide pharmaceutical development and toxicology studies a unique opportunity to revolutionize predictive drug toxicology assays and allow the creation of in vitro hepatic disease models.
Figures



Comment in
-
An identification of novel therapy for human hepatocellular carcinoma by using human induced pluripotent stem cells.Hepatology. 2010 Mar;51(3):1090-1. doi: 10.1002/hep.23418. Hepatology. 2010. PMID: 20041407 No abstract available.
References
-
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Dalgetty DM, Medine CN, Iredale JP, Hay DC. Progress and fuure challenges in stem cell-derived liver technologies. Am. J. Physiol. Gastrointest Liver Physiol. 2009;297 doi:10.1152/ajpgi.00138.2009. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
