Mechanism of DNA-protein cross-linking by chromium
- PMID: 19877617
- PMCID: PMC2822107
- DOI: 10.1021/tx9003402
Mechanism of DNA-protein cross-linking by chromium
Abstract
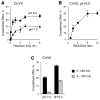
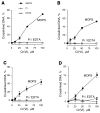
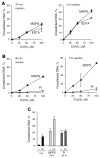
Hexavalent chromium is a known inducer of DNA-protein cross-links (DPCs) that contribute to repression of inducible genes and genotoxicity of this metal. Lymphocytic DPCs have also shown potential utility as biomarkers of human exposure to Cr(VI). Here, we examined the mechanism of DPC formation by Cr(VI) and the impact of its main cellular reducers. In vitro reactions of Cr(VI) with one-electron reducing thiols (glutathione and cysteine) or two-electron donating ascorbate were all efficient at DPC production, indicating a dispensable role of Cr(V). No Cr(VI) reducer was able to generate DPC in the presence of Cr(III)-chelating EDTA or phosphate. A critical role of Cr(III) in DNA-protein linkages was further confirmed by dissociation of Cr(VI)-induced DPC by phosphate. EDTA was very inefficient in DPC dissociation, indicating its poor suitability for testing of Cr(III)-mediated bridging and reversal of complex DPC. Reactions containing only one Cr-modified component (protein or DNA) showed that Cr(III)-DNA adduction was the initial step in DPC formation. Cross-linking proceeded slowly after the rapid formation of Cr-DNA adducts, indicating that protein conjugation was the rate-limiting step in DPC generation. Experiments with depletion of glutathione and restoration of ascorbate levels in human lung A549 cells showed that high cellular reducing capacity promotes DPC yield. Overall, our data provide evidence for a three-step cross-linking mechanism involving (i) reduction of Cr(VI) to Cr(III), (ii) Cr(III)-DNA binding, and (iii) protein capture by DNA-bound Cr(III) generating protein-Cr(III)-DNA cross-links.
Figures



Similar articles
-
Carcinogenic chromium(VI) induces cross-linking of vitamin C to DNA in vitro and in human lung A549 cells.Biochemistry. 2002 Mar 5;41(9):3156-67. doi: 10.1021/bi011942z. Biochemistry. 2002. PMID: 11863455
-
Chromium(VI) causes interstrand DNA cross-linking in vitro but shows no hypersensitivity in cross-link repair-deficient human cells.Chem Res Toxicol. 2013 Oct 21;26(10):1591-8. doi: 10.1021/tx400293s. Epub 2013 Oct 8. Chem Res Toxicol. 2013. PMID: 24059640 Free PMC article.
-
Analysis of EDTA-chelatable proteins from DNA-protein crosslinks induced by a carcinogenic chromium(VI) in cultured intact human cells.Mol Cell Biochem. 1999 Sep;199(1-2):149-62. doi: 10.1023/a:1006910732307. Mol Cell Biochem. 1999. PMID: 10544963
-
Chromium genotoxicity: A double-edged sword.Chem Biol Interact. 2010 Nov 5;188(2):276-88. doi: 10.1016/j.cbi.2010.04.018. Epub 2010 Apr 27. Chem Biol Interact. 2010. PMID: 20430016 Free PMC article. Review.
-
Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms.Mutat Res. 2003 Dec 10;533(1-2):3-36. doi: 10.1016/j.mrfmmm.2003.09.006. Mutat Res. 2003. PMID: 14643411 Review.
Cited by
-
XPA impacts formation but not proteasome-sensitive repair of DNA-protein cross-links induced by chromate.Mutagenesis. 2010 Jul;25(4):381-8. doi: 10.1093/mutage/geq017. Epub 2010 Apr 21. Mutagenesis. 2010. PMID: 20410141 Free PMC article.
-
Safety and efficacy of Availa®Cr (chromium chelate of DL-methionine) as a feed additive for dairy cows.EFSA J. 2020 Feb 26;18(2):e06026. doi: 10.2903/j.efsa.2020.6026. eCollection 2020 Feb. EFSA J. 2020. PMID: 32874235 Free PMC article.
-
Cytogenomics of hexavalent chromium (Cr 6+) exposed cells: a comprehensive review.Indian J Med Res. 2014 Mar;139(3):349-70. Indian J Med Res. 2014. PMID: 24820829 Free PMC article. Review.
-
Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double-strand breaks in human ovarian cancer cells.Mol Med Rep. 2016 Mar;13(3):2094-100. doi: 10.3892/mmr.2016.4763. Epub 2016 Jan 12. Mol Med Rep. 2016. PMID: 26782830 Free PMC article.
-
Variation in Extracellular Detoxification Is a Link to Different Carcinogenicity among Chromates in Rodent and Human Lungs.Chem Res Toxicol. 2017 Sep 18;30(9):1720-1729. doi: 10.1021/acs.chemrestox.7b00172. Epub 2017 Aug 20. Chem Res Toxicol. 2017. PMID: 28759204 Free PMC article.
References
-
- Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem Res Toxicol. 2005;18:3–11. - PubMed
-
- O’Brien T, Mandel GH, Pritchard DE, Patierno SR. Critical role of chromium(Cr)-DNA interactions in the formation of Cr-induced polymerase arresting lesions. Biochemistry. 2002;41:12529–12537. - PubMed
-
- Lay PA, Levina A. Activation of molecular oxygen during the reactions of chromium(VI/V/IV) with biological reductants: implications for chromium-induced genotoxicities. J Am Chem Soc. 1998;120:6704–6714.
-
- Hamilton JW, Wetterhahn KE. Chromium (VI)-induced DNA damage in chick embryo liver and blood cells in vivo. Carcinogenesis. 1986;7:2085–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

