Electron transfer from cytochrome c(2) to the reaction center: a transition state model for ionic strength effects due to neutral mutations
- PMID: 19877711
- PMCID: PMC2789999
- DOI: 10.1021/bi901332t
Electron transfer from cytochrome c(2) to the reaction center: a transition state model for ionic strength effects due to neutral mutations
Abstract
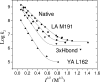
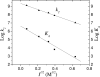
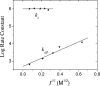
Interprotein electron transfer plays an important role in biological energy conversion. In this work, the electron transfer reaction between cytochrome c(2) (cyt) and the reaction center (RC) was studied to determine the mechanisms coupling association and electron transfer. Previous studies have shown that mutation of hydrophobic residues in the reaction interface, particularly Tyr L162, changes the binding affinity and rates of electron transfer at low ionic strengths. In this study, the effect of ionic strength on the second-order electron transfer rate constant, k(2), between cyt c(2) and native or mutant RCs was examined. Mutations of hydrophobic and hydrogen bonding residues caused k(2) to decrease more rapidly with an increase in ionic strength. This change is explained with a transition state model by a switch from a diffusion-limited reaction in native RCs, where electron transfer occurs upon each binding event, to a fast exchange reaction in the Tyr L162 mutant, where dissociation occurs before electron transfer and k(2) depends upon the equilibrium between bound and free protein complexes. The difference in ionic strength dependence is attributed to a smaller effect of ionic strength on the energy of the transition state compared to the bound state due to larger distances between charged residues in the transition state. This model explains the faster dissociation rate at higher ionic strengths that may assist rapid turnover that is important for biological function. These results provide a quantitative model for coupling protein association with electron transfer and elucidate the role of short-range interactions in determining the rate of electron transfer.
Figures


References
-
- Bendall D. In: Protein electron transfer. Bendall D, editor. Bios Scientific Publishers Ltd; Oxford, UK: 1996. pp. 43–68.
-
- Janin J. The kinetics of protein-protein recognition. Proteins: Structure, Function and Genetics. 1997;28:153–161. - PubMed
-
- Berg OG, von Hippel PH. Diffusion-controlled macromolecular interactions. Ann. Rev. Biophys. Biophys. Chem. 1985;14:131–160. - PubMed
-
- Onuchic JN, Beratan DN, Winkler JR, Gray HB. Pathway analysis of protein electron-transfer reactions. Annu. Rev. Biophys. Biomol. Struct. 1992;21:349–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

