Changes in Trypanosoma cruzi-specific immune responses after treatment: surrogate markers of treatment efficacy
- PMID: 19877967
- PMCID: PMC2805187
- DOI: 10.1086/648072
Changes in Trypanosoma cruzi-specific immune responses after treatment: surrogate markers of treatment efficacy
Abstract
Background: As many as 20 million people are living with Trypanosoma cruzi infection in Latin American, yet few receive any treatment. The major limitation in developing and evaluating potential new drugs for their efficacy is the lack of reliable tests to assess parasite burden and elimination.
Methods: Adults volunteers with chronic T. cruzi infection were evaluated clinically and stratified according to the Kuschnir classification. Individuals with group 0 and group 1 clinical status were treated with benznidazole (5 mg/kg per day for 30 days). The changes in T. cruzi-specific T cell and antibody responses, as well as in clinical status, were measured periodically over the 3-5-year follow-up period and were compared with pretreatment conditions and with values in an untreated control group.
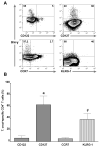
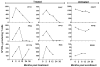
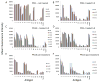
Results: The frequency of peripheral interferon (IFN)-gamma-producing T cells specific for T. cruzi declined as early as 12 months after benznidazole treatment and subsequently became undetectable in a substantial proportion of treated subjects. In addition, decreases in antibody responses to a pool of recombinant T. cruzi proteins also decreased in many of these same subjects. The shift to negative IFN-gamma T cell responses was highly associated with an early increase in IFN-gamma-producing T cells with phenotypic features of effector/effector memory cells in a subset of subjects. Benznidazole treatment also resulted in an increase in naive and early differentiated memory-like CD8(+) T cells in a majority of subjects.
Conclusions: Benznidazole treatment during chronic Chagas disease has a substantial impact on parasite-specific immune response that is likely indicative of treatment efficacy and cure.
Conflict of interest statement
No conflict of interest exists for all the authors involved in this study.
Figures
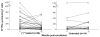


Comment in
-
New advances in the management of a long-neglected disease.Clin Infect Dis. 2009 Dec 1;49(11):1685-7. doi: 10.1086/648073. Clin Infect Dis. 2009. PMID: 19877968 No abstract available.
References
-
- Cancado JR. Tratamento especifico. In: Cancado JR, Chuster M, editors. Cardiopatía Chagásica. Belo Horizonte, Brazil: Fundacao Carlos Chagas; 1985. pp. 327–355.
-
- Fragata Filho AA, da Silva MA, Boainain E. Etiologic treatment of acute and chronic Chagas disease. Sao Paulo Med J. 1995;113:867–872. - PubMed
-
- Sgambatti de Andrade AL, Zicker F, de Oliveira RM, et al. Randomized trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. - PubMed
-
- Sosa Estani S, Segura EL, Ruiz AM, Velásquez E, Porcel BM, Yampotis C. Efficacy of treatment with benznidazole in children. Am J Trop Med Hyg. 1998;59:526–529. - PubMed
-
- Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas' disease with benznidazole: clinical and serologic evolution of patients with longterm follow-up. Am Heart J. 1994;127:151–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

