Viral entry mechanisms: the increasing diversity of paramyxovirus entry
- PMID: 19878307
- PMCID: PMC2795005
- DOI: 10.1111/j.1742-4658.2009.07401.x
Viral entry mechanisms: the increasing diversity of paramyxovirus entry
Abstract
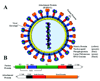
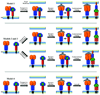
The paramyxovirus family contains established human pathogens such as the measles virus and human respiratory syncytial virus, as well as emerging pathogens including the Hendra and Nipah viruses and the recently identified human metapneumovirus. Two major envelope glycoproteins, the attachment protein and the fusion protein, promote the processes of viral attachment and virus-cell membrane fusion required for entry. Although common mechanisms of fusion protein proteolytic activation and the mechanism of membrane fusion promotion have been shown in recent years, considerable diversity exists in the family relating to receptor binding and the potential mechanisms of fusion triggering.
Figures

References
-
- Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott: Williams and Wilkins; 2007. pp. 1449–1496.
-
- Tong S, Compans RW. Alternative mechanisms of interaction between homotypic and heterotypic parainfluenza virus HN and F proteins. J. Gen. Virol. 1999;80:107–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

