Autoimmunity against type VII collagen in inflammatory bowel disease
- PMID: 19878366
- PMCID: PMC3823157
- DOI: 10.1111/j.1582-4934.2009.00959.x
Autoimmunity against type VII collagen in inflammatory bowel disease
Abstract
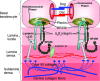
Autoimmunity against type VII collagen, an adhesion molecule of the extracellular matrix in epithelial basement membranes, is causing the rare organ-specific epidermolysis bullosa acquisita (EBA). An intriguing association between EBA and inflammatory bowel disease (IBD) has been extensively documented over the last decades, but, because of the very low incidence of EBA, received little attention from physicians involved in the care of patients with IBD. More recently, autoantibodies against type VII collagen have been detected in up to 68% of IBD patients. Although these findings suggest that chronic intestinal inflammation in IBD predisposes for autoimmunity against type VII collagen, their relevance for the pathogenesis of both IBD and EBA is still unclear. In this review article, the main features of the association between IBD and EBA are presented and pathomechanistic hypotheses as well as future lines of investigation in this area are discussed. Future research should provide new pathomechanistic insights and will likely facilitate the development of more specific and effective immunotherapeutic strategies for both conditions.
© 2009 The Authors Journal compilation © 2010 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. - PubMed
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. - PubMed
-
- Sitaru C, Goebeler M, Zillikens D. Bullous autoimmune dermatoses (I): pathogenesis and diagnosis. J Dtsch Dermatol Ges. 2004;2:123–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

