Macrophage cholesteryl ester mobilization and atherosclerosis
- PMID: 19878739
- PMCID: PMC2823947
- DOI: 10.1016/j.vph.2009.10.002
Macrophage cholesteryl ester mobilization and atherosclerosis
Abstract
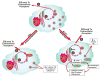
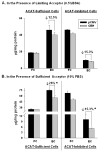
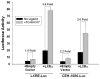
Accumulation of cholesteryl esters (CE) stored as cytoplasmic lipid droplets is the main characteristic of macrophage foam cells that are central to the development of atherosclerotic plaques. Since only unesterified or free cholesterol (FC) can be effluxed from the cells to extracellular cholesterol acceptors, hydrolysis of CE is the obligatory first step in CE mobilization from macrophages. This reaction, catalyzed by neutral cholesteryl ester hydrolase (CEH), is increasingly being recognized as the rate-limiting step in FC efflux. CEH, therefore, regulates the process of reverse cholesterol transport and ultimate elimination of cholesterol from the body. In this review, we summarize the earlier controversies surrounding the identity of CEH in macrophages, discuss the characteristics of the various candidates recognized to date and examine their role in mobilizing cellular CE and thus regulating atherogenesis. In addition, physiological requirements to hydrolyze lipid droplet-associated substrate and complexities of interfacial catalysis are also discussed to emphasize the importance of evaluating the biochemical characteristics of candidate enzymes that may be targeted in the future to attenuate atherosclerosis.
2009 Elsevier Inc. All rights reserved.
Figures


References
-
- Belfrage P, Fredrikson G, Olsson H, Strålfors P. Hormonal regulation of adipose tissue lipolysis by reversible phosphorylation of hormone-sensitive lipase. Prog Clin Biol Res. 1982;102(Pt C):213–223. - PubMed
-
- Belle V, Fournel A, Woudstra M, Ranaldi S, Prieri F, Thomé V, Currault J, Verger R, Guigliarelli B, Carrière F. Probing the opening of the pancreatic lipase lid using site-directed spin labeling and EPR spectroscopy. Biochemistry. 2007;46:2205–2214. - PubMed
-
- Bernard DW, Rodriguez A, Rothblat GH, Glick JM. cAMP stimulates cholesteryl ester clearance to high density lipoproteins in J7774 macrophages. J Biol Chem. 1991;266:710–716. - PubMed
-
- Bocan TM, Krause BR, Rosebury WS, Mueller SB, Lu X, Dagle C, Major T, Lathia C, Lee H. The ACAT inhibitor avasimbe reduces macrophages and matrix metalloproteinase expression in atherosclerotic lesion of hypercholesterolemic rabbits. Arteioscle Thromb Vasc Biol. 2000;20:70–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

