AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance
- PMID: 19878872
- PMCID: PMC2804470
- DOI: 10.1016/j.ccr.2009.09.028
AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance
Abstract
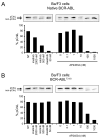
Inhibition of BCR-ABL by imatinib induces durable responses in many patients with chronic myeloid leukemia (CML), but resistance attributable to kinase domain mutations can lead to relapse and a switch to second-line therapy with nilotinib or dasatinib. Despite three approved therapeutic options, the cross-resistant BCR-ABL(T315I) mutation and compound mutants selected on sequential inhibitor therapy remain major clinical challenges. We report design and preclinical evaluation of AP24534, a potent, orally available multitargeted kinase inhibitor active against T315I and other BCR-ABL mutants. AP24534 inhibited all tested BCR-ABL mutants in cellular and biochemical assays, suppressed BCR-ABL(T315I)-driven tumor growth in mice, and completely abrogated resistance in cell-based mutagenesis screens. Our work supports clinical evaluation of AP24534 as a pan-BCR-ABL inhibitor for treatment of CML.
Figures

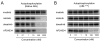



References
-
- Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O’Brien S, Garcia-Manero G, Giles F, Breeden M, Reeves N, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–4011. - PubMed
-
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

