Cell-based optimization of novel benzamides as potential antimalarial leads
- PMID: 19879133
- PMCID: PMC3532596
- DOI: 10.1016/j.bmcl.2009.10.050
Cell-based optimization of novel benzamides as potential antimalarial leads
Abstract
Screening our in-house compound collection using a cell based Plasmodium falciparum proliferation assay we discovered a known pan-kinase inhibitor scaffold as a hit. Further optimization of this series led us to a novel benzamide scaffold which was devoid of human kinase activity while retaining its antiplasmodial activity. The evolution of this compound series leading to optimized candidates with good cellular potency against multiple strains as well as decent in vivo profile is described in this Letter.
Figures

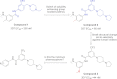
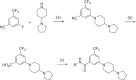


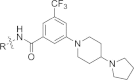
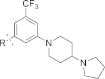
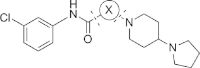

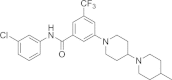
References
-
- WHO ‘World Malaria Report 2008’, June 5, 2009, http://apps.who.int/malaria/wmr2008/malaria2008.pdf.
-
- Noedl H., Se Y., Schaecher K., Smith B.L., Socheat D., Fukuda M. New Eng. J. Med. 2008;359:2619. - PubMed
-
- Wu, T.; Nagle, A.; Sakata, T.; Deng, X.; Chen, Z.; Henson, K.; Chang, J.; Plouffe, D.; Tuntland, T.; Kuhen, K.; Winzeler, E.; Gray, N.; Chatterjee, A. K. Abstract of Papers, 236th National Meeting of the American Chemical Society, Philadelphia, PA, August 2008. MEDI-339.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

