Deviant kinetochore microtubule dynamics underlie chromosomal instability
- PMID: 19879145
- PMCID: PMC2787757
- DOI: 10.1016/j.cub.2009.09.055
Deviant kinetochore microtubule dynamics underlie chromosomal instability
Abstract
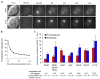
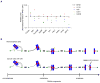
The persistent malattachment of microtubules to chromosomes at kinetochores is a major mechanism of chromosomal instability (CIN) [1, 2]. In normal diploid cells, malattachments arise spontaneously and are efficiently corrected to preserve genomic stability [3]. However, it is unknown whether cancer cells with CIN possess the ability to efficiently correct attachment errors. Here we show that kinetochore microtubule attachments in cancer cells with CIN are inherently more stable than those in normal diploid RPE-1 cells. The observed differences in attachment stability account for the persistence of malattachments into anaphase, where they cause chromosome missegregation. Furthermore, increasing the stability of kinetochore microtubule attachments in normal diploid RPE-1 cells, either by depleting the tumor suppressor protein APC or the kinesin-13 protein MCAK, is sufficient to promote chromosome segregation defects to levels comparable to those in cancer cells with CIN. Collectively, these data identify that cancer cells have a diminished capacity to correct erroneous kinetochore microtubule attachments and account for the widespread occurrence of CIN in tumors [4].
Figures
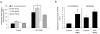
Comment in
-
Mitosis: too much of a good thing (can be bad).Curr Biol. 2009 Dec 1;19(22):R1032-4. doi: 10.1016/j.cub.2009.10.013. Curr Biol. 2009. PMID: 19948139 Free PMC article.
References
-
- Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci. 2003;116:4213–4225. - PubMed
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. - PubMed
-
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nature Rev Mol Cell Biol. 2004;5:45–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

