Myosin II dynamics are regulated by tension in intercalating cells
- PMID: 19879198
- PMCID: PMC2854079
- DOI: 10.1016/j.devcel.2009.09.003
Myosin II dynamics are regulated by tension in intercalating cells
Abstract
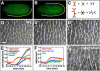
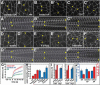
Axis elongation in Drosophila occurs through polarized cell rearrangements driven by actomyosin contractility. Myosin II promotes neighbor exchange through the contraction of single cell boundaries, while the contraction of myosin II structures spanning multiple pairs of cells leads to rosette formation. Here we show that multicellular actomyosin cables form at a higher frequency than expected by chance, indicating that cable assembly is an active process. Multicellular cables are sites of increased mechanical tension as measured by laser ablation. Fluorescence recovery after photobleaching experiments show that myosin II is stabilized at the cortex in regions of increased tension. Myosin II is recruited in response to an ectopic force and relieving tension leads to a rapid loss of myosin, indicating that tension is necessary and sufficient for cortical myosin localization. These results demonstrate that myosin II dynamics are regulated by tension in a positive feedback loop that leads to multicellular actomyosin cable formation and efficient tissue elongation.
Figures


References
-
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. - PubMed
-
- Blankenship J, Backovic S, Sanny J, Weitz O, Zallen J. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. - PubMed
-
- Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol. 2009;11:859–864. - PubMed
-
- Cremo C, Geeves M. Interaction of actin and ADP with the head domain of smooth muscle myosin: implications for strain-dependent ADP release in smooth muscle. Biochemistry. 1998;37:1969–1978. - PubMed
-
- Dawes-Hoang R, Parmar K, Christiansen A, Phelps C, Brand A, Wieschaus E. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

