Evaluating the role of carbonic anhydrases in the transport of HCO3--related species
- PMID: 19879980
- PMCID: PMC4699190
- DOI: 10.1016/j.bbapap.2009.10.021
Evaluating the role of carbonic anhydrases in the transport of HCO3--related species
Abstract
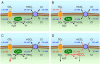
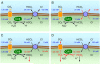
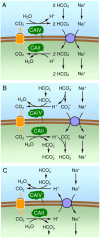
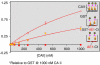
The soluble enzyme carbonic anhydrase II (CAII) plays an important role in CO(2) influx and efflux by red blood cells (RBCs), a process initiated by changes in the extracellular [CO(2)] (CO(2)-initiated CO(2) transport). Evidence suggests that CAII may be part of a macromolecular complex at the inner surface of the RBC membrane. Some have suggested CAII specifically binds to a motif on the cytoplasmic C terminus (Ct) of the Cl-HCO(3) exchanger AE1 and some other members of the SLC4 family of HCO(3)(-) transporters, a transport metabolon. Moreover, others have suggested that this bound CAII enhances the transport of HCO(3)(-)-related species-HCO(3)(-), CO(3)(), or CO(3)() ion pairs-when the process is initiated by altering the activity of the transporter (HCO(3)(-)-initiated HCO(3)(-) transport). In this review, I assess the theoretical roles of CAs in the transport of CO(2) and HCO(3)(-)-related species, concluding that although the effect of bound CAII on CO(2)-initiated CO(2) transport is expected to be substantial, the effect of bound CAs on HCO(3)(-)-initiated HCO(3)(-) transport is expected to be modest at best. I also assess the experimental evidence for CAII binding to AE1 and other transporters, and the effects of this binding on HCO(3)(-)-initiated HCO(3)(-) transport. The early conclusion that CAII binds to the Ct of AE1 appears to be the result of unpredictable effects of GST in the GST fusion proteins used in the studies. The early conclusion that bound CAII speeds HCO(3)(-)-initiated HCO(3)(-) transport appears to be the result of CAII accelerating the pH changes used as a read-out of transport. Thus, it appears that CAII does not bind directly to AE1 or other SLC4 proteins, and that bound CAII does not substantially accelerate HCO(3)(-)-initiated HCO(3)(-) transport.
Copyright 2009. Published by Elsevier B.V.
Figures




Similar articles
-
An intramolecular transport metabolon: fusion of carbonic anhydrase II to the COOH terminus of the Cl(-)/HCO(3)(-)exchanger, AE1.Am J Physiol Cell Physiol. 2011 Aug;301(2):C336-46. doi: 10.1152/ajpcell.00005.2011. Epub 2011 May 4. Am J Physiol Cell Physiol. 2011. PMID: 21543742
-
The bicarbonate transport metabolon.J Enzyme Inhib Med Chem. 2004 Jun;19(3):231-6. doi: 10.1080/14756360410001704443. J Enzyme Inhib Med Chem. 2004. PMID: 15499994 Review.
-
Carbonic anhydrase: in the driver's seat for bicarbonate transport.JOP. 2001 Jul;2(4 Suppl):165-70. JOP. 2001. PMID: 11875254 Review.
-
Identification of the carbonic anhydrase II binding site in the Cl(-)/HCO(3)(-) anion exchanger AE1.Biochemistry. 2000 May 9;39(18):5527-33. doi: 10.1021/bi992564p. Biochemistry. 2000. PMID: 10820026
-
Localization of the Cl-/HCO3- anion exchanger binding site to the amino-terminal region of carbonic anhydrase II.Biochemistry. 2000 Nov 7;39(44):13344-9. doi: 10.1021/bi0015111. Biochemistry. 2000. PMID: 11063570
Cited by
-
Exploring new structural features of the 4-[(3-methyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene)amino]benzenesulphonamide scaffold for the inhibition of human carbonic anhydrases.J Enzyme Inhib Med Chem. 2019 Dec;34(1):1526-1533. doi: 10.1080/14756366.2019.1654470. J Enzyme Inhib Med Chem. 2019. PMID: 31431095 Free PMC article.
-
Cation-coupled bicarbonate transporters.Compr Physiol. 2014 Oct;4(4):1605-37. doi: 10.1002/cphy.c130005. Compr Physiol. 2014. PMID: 25428855 Free PMC article. Review.
-
Structural and biophysical characterization of the α-carbonic anhydrase from the gammaproteobacterium Thiomicrospira crunogena XCL-2: insights into engineering thermostable enzymes for CO2 sequestration.Acta Crystallogr D Biol Crystallogr. 2015 Aug;71(Pt 8):1745-56. doi: 10.1107/S1399004715012183. Epub 2015 Jul 31. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26249355 Free PMC article.
-
Zebrafish and mouse TASK-2 K(+) channels are inhibited by increased CO2 and intracellular acidification.Pflugers Arch. 2014 Jul;466(7):1317-27. doi: 10.1007/s00424-013-1365-2. Epub 2013 Oct 1. Pflugers Arch. 2014. PMID: 24081451
-
Local pH domains regulate NHE3-mediated Na⁺ reabsorption in the renal proximal tubule.Am J Physiol Renal Physiol. 2014 Dec 1;307(11):F1249-62. doi: 10.1152/ajprenal.00174.2014. Epub 2014 Oct 8. Am J Physiol Renal Physiol. 2014. PMID: 25298526 Free PMC article.
References
-
- Garrels RM, Thompson ME, Siever R. Control of carbonate solubility by carbonate complexes. Am. J. Sci. 1961;259:24–45.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

