Signaling pathways induced by a tumor-derived vaccine in antigen presenting cells
- PMID: 19880213
- PMCID: PMC2881173
- DOI: 10.1016/j.imbio.2009.09.006
Signaling pathways induced by a tumor-derived vaccine in antigen presenting cells
Abstract
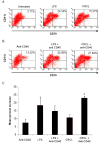
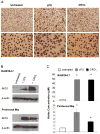
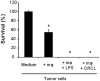
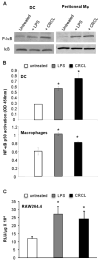
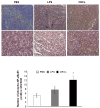
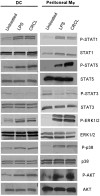
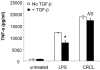
We have previously reported on the anti-tumoral potential of a chaperone-rich cell lysate (CRCL) vaccine. Immunization with CRCL generated from tumors elicits specific T and NK cell-dependent immune responses leading to protective immunity in numerous mouse tumor models. CRCL provides both a source of tumor antigens and danger signals leading to dendritic cell activation. In humans, tumor-derived CRCL induces dendritic cell activation and CRCL-loaded dendritic cells promote the generation of cytotoxic T lymphocytes in vitro. The current study was designed to identify the signaling events and modifications triggered by CRCL in antigen presenting cells. Our results indicate that tumor-derived CRCL not only promotes the activation of dendritic cells, but also significantly fosters the function of macrophages that thus appear as major targets of this vaccine. Activation of both cell types is associated with the induction of the MAP kinase pathway, the phosphorylation of STAT1, STAT5 and AKT and with transcription factor NF-kappaB activation in vitro and in vivo. These results thus provide important insights into the mechanisms by which CRCL-based vaccines exert their adjuvant effects on antigen presenting cells.
Copyright 2009 Elsevier GmbH. All rights reserved.
Figures
Similar articles
-
Exosomes from Dendritic Cells Loaded with Chaperone-Rich Cell Lysates Elicit a Potent T Cell Immune Response Against Intracranial Glioma in Mice.J Mol Neurosci. 2015 Jul;56(3):631-43. doi: 10.1007/s12031-015-0506-9. Epub 2015 Feb 14. J Mol Neurosci. 2015. PMID: 25680514
-
Differential capacity of chaperone-rich lysates in cross-presenting human endogenous and exogenous melanoma differentiation antigens.Int J Hyperthermia. 2008 Dec;24(8):623-37. doi: 10.1080/02656730802213384. Int J Hyperthermia. 2008. PMID: 18608582
-
Induction of BCR-ABL-specific immunity following vaccination with chaperone-rich cell lysates derived from BCR-ABL+ tumor cells.Blood. 2005 Mar 1;105(5):2016-22. doi: 10.1182/blood-2004-05-1915. Epub 2004 Sep 16. Blood. 2005. PMID: 15374884 Free PMC article.
-
Chaperone-rich cell lysates, immune activation and tumor vaccination.Cancer Immunol Immunother. 2006 Mar;55(3):329-38. doi: 10.1007/s00262-005-0694-1. Epub 2005 May 11. Cancer Immunol Immunother. 2006. PMID: 15887013 Free PMC article. Review.
-
Tumor cell lysates as immunogenic sources for cancer vaccine design.Hum Vaccin Immunother. 2014;10(11):3261-9. doi: 10.4161/21645515.2014.982996. Hum Vaccin Immunother. 2014. PMID: 25625929 Free PMC article. Review.
Cited by
-
HSP90 multi-functionality in cancer.Front Immunol. 2024 Aug 1;15:1436973. doi: 10.3389/fimmu.2024.1436973. eCollection 2024. Front Immunol. 2024. PMID: 39148727 Free PMC article. Review.
-
Th-1 lymphocytes induce dendritic cell tumor killing activity by an IFN-γ-dependent mechanism.J Immunol. 2011 Dec 15;187(12):6310-7. doi: 10.4049/jimmunol.1101812. Epub 2011 Nov 9. J Immunol. 2011. PMID: 22075702 Free PMC article.
-
Exosomes from Dendritic Cells Loaded with Chaperone-Rich Cell Lysates Elicit a Potent T Cell Immune Response Against Intracranial Glioma in Mice.J Mol Neurosci. 2015 Jul;56(3):631-43. doi: 10.1007/s12031-015-0506-9. Epub 2015 Feb 14. J Mol Neurosci. 2015. PMID: 25680514
-
Endoplasmic reticulum chaperones and their roles in the immunogenicity of cancer vaccines.Front Oncol. 2015 Jan 6;4:379. doi: 10.3389/fonc.2014.00379. eCollection 2014. Front Oncol. 2015. PMID: 25610811 Free PMC article. Review.
-
PIAS1 and STAT-3 impair the tumoricidal potential of IFN-γ-stimulated mouse dendritic cells generated with IL-15.Eur J Immunol. 2014 Aug;44(8):2489-2499. doi: 10.1002/eji.201343803. Epub 2014 May 23. Eur J Immunol. 2014. PMID: 24777831 Free PMC article.
References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. - PubMed
-
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. - PubMed
-
- Bauer PM, Buga GM, Fukuto JM, Pegg AE, Ignarro LJ. Nitric oxide inhibits ornithine decarboxylase via S-nitrosylation of cysteine 360 in the active site of the enzyme. J Biol Chem. 2001;276:34458–34464. - PubMed
-
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

