Trans-sialidase activity of Photobacterium damsela alpha2,6-sialyltransferase and its application in the synthesis of sialosides
- PMID: 19880425
- PMCID: PMC2800248
- DOI: 10.1093/glycob/cwp172
Trans-sialidase activity of Photobacterium damsela alpha2,6-sialyltransferase and its application in the synthesis of sialosides
Abstract
Trans-sialidases catalyze the transfer of a sialic acid from one sialoside to an acceptor to form a new sialoside. alpha2,3-Trans-sialidase activity was initially discovered in the parasitic protozoan Trypanosoma cruzi, and more recently was found in a multifunctional Pasteurella multocida sialyltransferase PmST1. alpha2,8-Trans-sialidase activity was also described for a multifunctional Campylobacter jejuni sialyltransferase CstII. We report here the discovery of the alpha2,6-trans-sialidase activity of a previously reported recombinant truncated bacterial alpha2,6-sialyltransferase from Photobacterium damsela (Delta15Pd2,6ST). This is the first time that the alpha2,6-trans-sialidase activity has ever been identified. Kinetic studies indicate that Delta15Pd2,6ST-catalyzed trans-sialidase reaction follows a ping-pong bi-bi reaction mechanism. Cytidine 5'-monophosphate, the product of sialyltransferase reactions, is not required by the trans-sialidase activity of the enzyme but enhances the trans-sialidase activity modestly as a non-essential activator. Using chemically synthesized Neu5AcalphapNP and LacbetaMU, alpha2,6-linked sialoside Neu5Acalpha2,6LacbetaMU has been obtained in one-step in high yield using the trans-sialidase activity of Delta15Pd2,6ST. In addition to the alpha2,6-trans-sialidase activity, Delta15Pd2,6ST also has alpha2,6-sialidase activity. The multifunctionality is thus a common feature of many bacterial sialyltransferases.
Figures
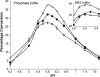

 ) 2.5 mM; (○) 5 mM; and (▴) 10 mM. The solid lines corresponded to data fitted to Eq. (1).
) 2.5 mM; (○) 5 mM; and (▴) 10 mM. The solid lines corresponded to data fitted to Eq. (1).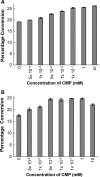

 ) 0 mM; (□) 1 mM; (▴) 10 mM. The solid lines corresponded to data fitted to Eq. (2) (data obtained in the presence of 10 mM CMP did not fit).
) 0 mM; (□) 1 mM; (▴) 10 mM. The solid lines corresponded to data fitted to Eq. (2) (data obtained in the presence of 10 mM CMP did not fit).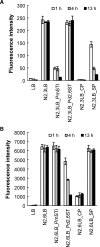

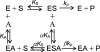
Similar articles
-
Multifunctionality of Campylobacter jejuni sialyltransferase CstII: characterization of GD3/GT3 oligosaccharide synthase, GD3 oligosaccharide sialidase, and trans-sialidase activities.Glycobiology. 2008 Sep;18(9):686-97. doi: 10.1093/glycob/cwn047. Epub 2008 May 28. Glycobiology. 2008. PMID: 18509108 Free PMC article.
-
Decreasing the sialidase activity of multifunctional Pasteurella multocida α2-3-sialyltransferase 1 (PmST1) by site-directed mutagenesis.Mol Biosyst. 2011 Nov;7(11):3021-7. doi: 10.1039/c1mb05182b. Epub 2011 Aug 19. Mol Biosyst. 2011. PMID: 21858283 Free PMC article.
-
N-Terminal 112 amino acid residues are not required for the sialyltransferase activity of Photobacterium damsela alpha2,6-sialyltransferase.Biotechnol Lett. 2008 Apr;30(4):671-6. doi: 10.1007/s10529-007-9588-y. Epub 2007 Nov 8. Biotechnol Lett. 2008. PMID: 17989925 Free PMC article.
-
Marine bacterial sialyltransferases.Mar Drugs. 2010 Nov 5;8(11):2781-94. doi: 10.3390/md8112781. Mar Drugs. 2010. PMID: 21139844 Free PMC article. Review.
-
Trans-sialidase: a unique enzyme activity discovered in the protozoan Trypanosoma cruzi.FASEB J. 1993 Oct;7(13):1257-64. doi: 10.1096/fasebj.7.13.8405811. FASEB J. 1993. PMID: 8405811 Review.
Cited by
-
Constructing a human complex type N-linked glycosylation pathway in Kluyveromyces marxianus.PLoS One. 2020 May 29;15(5):e0233492. doi: 10.1371/journal.pone.0233492. eCollection 2020. PLoS One. 2020. PMID: 32469948 Free PMC article.
-
Enabling Chemoenzymatic Strategies and Enzymes for Synthesizing Sialyl Glycans and Sialyl Glycoconjugates.Acc Chem Res. 2024 Jan 16;57(2):234-246. doi: 10.1021/acs.accounts.3c00614. Epub 2023 Dec 21. Acc Chem Res. 2024. PMID: 38127793 Free PMC article.
-
Synthesis of selective inhibitors against V. cholerae sialidase and human cytosolic sialidase NEU2.Org Biomol Chem. 2012 Aug 14;10(30):6112-20. doi: 10.1039/c2ob25335f. Epub 2012 May 29. Org Biomol Chem. 2012. PMID: 22641268 Free PMC article.
-
A comparative study of the substrate preference of the sialidases, CpNanI, HpNanH, and BbSia2 towards 2-Aminobenzamide-labeled 3'-Sialyllactose, 6'-Sialyllactose, and Sialyllacto-N-tetraose-b.Biochem Biophys Rep. 2024 Jul 19;39:101791. doi: 10.1016/j.bbrep.2024.101791. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 39156723 Free PMC article.
-
Synthesis of Human Milk Oligosaccharides: Protein Engineering Strategies for Improved Enzymatic Transglycosylation.Molecules. 2019 May 28;24(11):2033. doi: 10.3390/molecules24112033. Molecules. 2019. PMID: 31141914 Free PMC article. Review.
References
-
- Amaya MF, Watts AG, Damager I, Wehenkel A, Nguyen T, Buschiazzo A, Paris G, Frasch AC, Withers SG, Alzari PM. Structural insights into the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Structure. 2004;12:775–784. - PubMed
-
- Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary persepective. Chem Rev. 2002;102:439–469. - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. - PubMed
-
- Bern C, Montgomery SP, Katz L, Caglioti S, Stramer SL. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21:476–482. - PubMed
-
- Buschiazzo A, Amaya MF, Cremona ML, Frasch AC, Alzari PM. The crystal structure and mode of action of trans-sialidase, a key enzyme in Trypanosoma cruzi pathogenesis. Mol Cell. 2002;10:757–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

