Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice
- PMID: 19880489
- PMCID: PMC2798862
- DOI: 10.1182/blood-2009-08-232967
Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice
Abstract
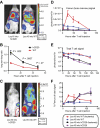
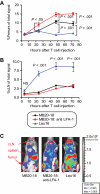
We have established a model of leukemia immunotherapy using T cells expressing chimeric T-cell receptors (cTCRs) targeting the CD20 molecule expressed on normal and neoplastic B cells. After transfer into human CD20 (hCD20) transgenic mice, cTCR(+) T cells showed antigen-specific delayed egress from the lungs, concomitant with T-cell deletion. Few cTCR(+) T cells reached the bone marrow (BM) in hCD20 transgenic mice, precluding effectiveness against leukemia. Anti-hCD20 antibody-mediated B-cell depletion before adoptive T-cell therapy permitted egress of mouse CD20-specific cTCR(+) T cells from the lungs, enhanced T-cell survival, and promoted cTCR(+) T cell-dependent elimination of established mouse CD20(+) leukemia. Furthermore, CD20-specific cTCR(+) T cells eliminated residual B cells refractory to depletion with monoclonal antibodies. These findings suggest that combination of antibody therapy that depletes antigen-expressing normal tissues with adoptive T-cell immunotherapy enhances the ability of cTCR(+) T cells to survive and control tumors.
Figures


References
-
- Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18(8):712–725. - PubMed
-
- Wang J, Press OW, Lindgren CG, et al. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes. Mol Ther. 2004;9(4):577–586. - PubMed
-
- Jensen MC, Clarke P, Tan G, et al. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000;1(1):49–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

