Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia
- PMID: 19880498
- PMCID: PMC2826761
- DOI: 10.1182/blood-2009-05-218560
Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia
Abstract
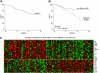
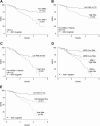
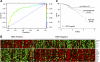
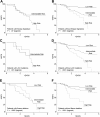
To determine whether gene expression profiling could improve outcome prediction in children with acute lymphoblastic leukemia (ALL) at high risk for relapse, we profiled pretreatment leukemic cells in 207 uniformly treated children with high-risk B-precursor ALL. A 38-gene expression classifier predictive of relapse-free survival (RFS) could distinguish 2 groups with differing relapse risks: low (4-year RFS, 81%, n = 109) versus high (4-year RFS, 50%, n = 98; P < .001). In multivariate analysis, the gene expression classifier (P = .001) and flow cytometric measures of minimal residual disease (MRD; P = .001) each provided independent prognostic information. Together, they could be used to classify children with high-risk ALL into low- (87% RFS), intermediate- (62% RFS), or high- (29% RFS) risk groups (P < .001). A 21-gene expression classifier predictive of end-induction MRD effectively substituted for flow MRD, yielding a combined classifier that could distinguish these 3 risk groups at diagnosis (P < .001). These classifiers were further validated on an independent high-risk ALL cohort (P = .006) and retainedindependent prognostic significance (P < .001) in the presence of other recently described poor prognostic factors (IKAROS/IKZF1 deletions, JAK mutations, and kinase expression signatures). Thus, gene expression classifiers improve ALL risk classification and allow prospective identification of children who respond or fail current treatment regimens. These trials were registered at http://clinicaltrials.gov under NCT00005603.
Figures

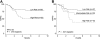
Comment in
-
Pediatric oncology: Gene-expression profiling is prognostic in ALL cases.Nat Rev Clin Oncol. 2010 May;7(5):239. doi: 10.1038/nrclinonc.2010.58. Nat Rev Clin Oncol. 2010. PMID: 20432522 No abstract available.
References
-
- Pui CH, Evans WE. Drug therapy: treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. - PubMed
-
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukemia. Lancet. 2008;371:1030–1043. - PubMed
-
- Pui CH, Pei DQ, Sandlund JT, et al. Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:7936–7941. - PubMed
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

