SHIP2 (SH2 domain-containing inositol phosphatase 2) SH2 domain negatively controls SHIP2 monoubiquitination in response to epidermal growth factor
- PMID: 19880507
- PMCID: PMC2794722
- DOI: 10.1074/jbc.M109.064923
SHIP2 (SH2 domain-containing inositol phosphatase 2) SH2 domain negatively controls SHIP2 monoubiquitination in response to epidermal growth factor
Abstract
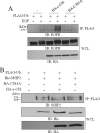
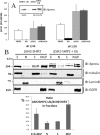
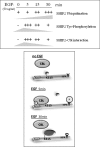
The SH2 domain containing inositol 5-phosphatase SHIP2 contains several interacting domains that are important for scaffolding properties. We and others have previously reported that SHIP2 interacts with the E3 ubiquitin ligase c-Cbl. Here, we identified human SHIP2 monoubiquitination on lysine 315. SHIP2 could also be polyubiquitinated but was not degraded by the 26 S proteasome. Furthermore, we identified a ubiquitin-interacting motif at the C-terminal end of SHIP2 that confers ubiquitin binding capacity. However, this ubiquitin-interacting motif is dispensable for its monoubiquitination. We showed that neither c-Cbl nor Nedd4-1 play the role of ubiquitin ligase for SHIP2. Strikingly, monoubiquitination of the DeltaSH2-SHIP2 mutant (lacking the N-terminal SH2 domain) is strongly increased, suggesting an intrinsic inhibitory effect of the SHIP2 SH2 domain on its monoubiquitination. Moreover, SHIP2 monoubiquitination was increased upon 30 min of epidermal growth factor stimulation. This correlates with the loss of interaction between the SHIP2 SH2 domain and c-Cbl. In this model, c-Cbl could mask the monoubiquitination site and thereby prevent SHIP2 monoubiquitination. The present study thus reveals an unexpected and novel role of SHIP2 SH2 domain in the regulation of its newly identified monoubiquitination.
Figures






Similar articles
-
Down-regulation of active ACK1 is mediated by association with the E3 ubiquitin ligase Nedd4-2.J Biol Chem. 2009 Mar 20;284(12):8185-94. doi: 10.1074/jbc.M806877200. Epub 2009 Jan 14. J Biol Chem. 2009. PMID: 19144635 Free PMC article.
-
Protein phosphatase 2A PR130/B''alpha1 subunit binds to the SH2 domain-containing inositol polyphosphate 5-phosphatase 2 and prevents epidermal growth factor (EGF)-induced EGF receptor degradation sustaining EGF-mediated signaling.FASEB J. 2010 Feb;24(2):538-47. doi: 10.1096/fj.09-140228. Epub 2009 Oct 13. FASEB J. 2010. PMID: 19825976
-
The association between the SH2-containing inositol polyphosphate 5-Phosphatase 2 (SHIP2) and the adaptor protein APS has an impact on biochemical properties of both partners.J Cell Physiol. 2008 Jan;214(1):260-72. doi: 10.1002/jcp.21193. J Cell Physiol. 2008. PMID: 17620296
-
SHIP2 multiple functions: a balance between a negative control of PtdIns(3,4,5)P₃ level, a positive control of PtdIns(3,4)P₂ production, and intrinsic docking properties.J Cell Biochem. 2011 Sep;112(9):2203-9. doi: 10.1002/jcb.23146. J Cell Biochem. 2011. PMID: 21503961 Review.
-
The SH2 domain containing inositol polyphosphate 5-phosphatase-2: SHIP2.Int J Biochem Cell Biol. 2005 Nov;37(11):2260-5. doi: 10.1016/j.biocel.2005.05.003. Int J Biochem Cell Biol. 2005. PMID: 15964236 Review.
Cited by
-
Inducible STAT3 NH2 terminal mono-ubiquitination promotes BRD4 complex formation to regulate apoptosis.Cell Signal. 2014 Jul;26(7):1445-55. doi: 10.1016/j.cellsig.2014.03.007. Epub 2014 Mar 20. Cell Signal. 2014. PMID: 24657799 Free PMC article.
-
A synthetic polyphosphoinositide headgroup surrogate in complex with SHIP2 provides a rationale for drug discovery.ACS Chem Biol. 2012 May 18;7(5):822-8. doi: 10.1021/cb200494d. Epub 2012 Feb 27. ACS Chem Biol. 2012. PMID: 22330088 Free PMC article.
-
Downregulation of SHIP2 by Hepatitis B Virus X Promotes the Metastasis and Chemoresistance of Hepatocellular Carcinoma through SKP2.Cancers (Basel). 2019 Jul 27;11(8):1065. doi: 10.3390/cancers11081065. Cancers (Basel). 2019. PMID: 31357665 Free PMC article.
-
Emerging role of Lys-63 ubiquitination in protein kinase and phosphatase activation and cancer development.Oncogene. 2010 Aug 12;29(32):4493-503. doi: 10.1038/onc.2010.190. Epub 2010 Jun 7. Oncogene. 2010. PMID: 20531303 Free PMC article. Review.
References
-
- Ooms L. M., Horan K. A., Rahman P., Seaton G., Gurung R., Kethesparan D. S., Mitchell C. A. (2009) Biochem. J. 419, 29–49 - PubMed
-
- Pesesse X., Deleu S., De Smedt F., Drayer L., Erneux C. (1997) Biochem. Biophys. Res. Commun. 239, 697–700 - PubMed
-
- Ishihara H., Sasaoka T., Hori H., Wada T., Hirai H., Haruta T., Langlois W. J., Kobayashi M. (1999) Biochem. Biophys. Res. Commun. 260, 265–272 - PubMed
-
- Pesesse X., Dewaste V., De Smedt F., Laffargue M., Giuriato S., Moreau C., Payrastre B., Erneux C. (2001) J. Biol. Chem. 276, 28348–28355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

