Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization-driven process
- PMID: 19880508
- PMCID: PMC2801264
- DOI: 10.1074/jbc.M109.049924
Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization-driven process
Abstract
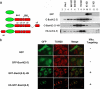
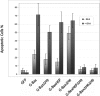
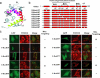
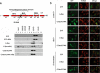
The apoptosis gateway protein Bax normally exists in the cytosol as a globular shaped monomer composed of nine alpha-helices. During apoptosis, Bax translocates to the mitochondria, forms homo-oligomers, and subsequently induces mitochondrial damage. The mechanism of Bax mitochondrial translocation remains unclear. Among the nine alpha-helices of Bax, helices 4, 5, 6, and 9 are capable of targeting a heterologous protein to mitochondria. However, only helices 6 and 9 can independently direct the oligomerized Bax to the mitochondria. Although Bax mitochondrial translocation can still proceed with mutations in either helix 6 or helix 9, combined mutations completely abolished mitochondrial targeting in response to activating signals. Using a proline mutagenesis scanning analysis, we demonstrated that conformational changes were sufficient to cause Bax to move from the cytosol to the mitochondria. Moreover, we found that homo-oligomerization of Bax contributed to its mitochondrial translocation. These results suggest that Bax is targeted to the mitochondria through the exposure of one or both of the two functional mitochondrial targeting sequences in a conformational change-driven and homo-oligomerization-aided process.
Figures


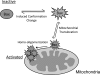
References
-
- Danial N. N., Korsmeyer S. J. (2004) Cell 116, 205–219 - PubMed
-
- Cory S., Adams J. M. (2002) Nat. Rev. Cancer 2, 647–656 - PubMed
-
- Green D. R., Kroemer G. (2004) Science 305, 626–629 - PubMed
-
- Adams J. M. (2003) Genes Dev. 17, 2481–2495 - PubMed
-
- Jiang X., Wang X. (2004) Annu. Rev. Biochem. 73, 87–106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

