RNA interference in Trypanosoma brucei: role of the n-terminal RGG domain and the polyribosome association of argonaute
- PMID: 19880512
- PMCID: PMC2794767
- DOI: 10.1074/jbc.M109.073072
RNA interference in Trypanosoma brucei: role of the n-terminal RGG domain and the polyribosome association of argonaute
Abstract
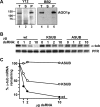
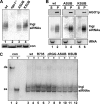
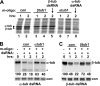
Argonaute proteins (AGOs) are central to RNA interference (RNAi) and related silencing pathways. At the core of the RNAi pathway in the ancient parasitic eukaryote Trypanosoma brucei is a single Argonaute protein, TbAGO1, with an established role in the destruction of potentially harmful retroposon transcripts. One notable feature of TbAGO1 is that a fraction sediments with polyribosomes, and this association is facilitated by an arginine/glycine-rich domain (RGG domain) at the N terminus of the protein. Here we report that reducing the size of the RGG domain and, in particular, mutating all arginine residues severely reduced the association of TbAGO1 with polyribosomes and RNAi-induced cleavage of mRNA. However, these mutations did not change the cellular localization of Argonaute and did not affect the accumulation of single-stranded siRNAs, an essential step in the activation of the RNA-induced silencing complex. We further show that mRNA on polyribosomes can be targeted for degradation, although this alliance is not a pre-requisite. Finally, sequestering tubulin mRNAs from translation with antisense morpholino oligonucleotides reduced the RNAi response indicating that mRNAs not engaged in translation may be less accessible to the RNAi machinery. We conclude that the association of the RNAi machinery and target mRNA on polyribosomes promotes an efficient RNAi response. This mechanism may represent an ancient adaptation to ensure that retroposon transcripts are efficiently destroyed, if they become associated with the translational apparatus.
Figures






Similar articles
-
Function of the Trypanosome Argonaute 1 protein in RNA interference requires the N-terminal RGG domain and arginine 735 in the Piwi domain.J Biol Chem. 2004 Nov 26;279(48):49889-93. doi: 10.1074/jbc.M409280200. Epub 2004 Sep 21. J Biol Chem. 2004. PMID: 15383544
-
Depletion of newly synthesized Argonaute1 impairs the RNAi response in Trypanosoma brucei.RNA. 2007 Jul;13(7):1132-9. doi: 10.1261/rna.474707. Epub 2007 May 25. RNA. 2007. PMID: 17526643 Free PMC article.
-
TbAGO1, an argonaute protein required for RNA interference, is involved in mitosis and chromosome segregation in Trypanosoma brucei.BMC Biol. 2003 Dec 12;1:2. doi: 10.1186/1741-7007-1-2. BMC Biol. 2003. PMID: 14670085 Free PMC article.
-
An RNAi screen of the RRM-domain proteins of Trypanosoma brucei.Mol Biochem Parasitol. 2009 Jan;163(1):61-5. doi: 10.1016/j.molbiopara.2008.09.001. Epub 2008 Sep 18. Mol Biochem Parasitol. 2009. PMID: 18840477
-
RNA interference: advances and questions.Philos Trans R Soc Lond B Biol Sci. 2002 Jan 29;357(1417):65-70. doi: 10.1098/rstb.2001.0952. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 11839183 Free PMC article. Review.
Cited by
-
Small interfering RNA-mediated translation repression alters ribosome sensitivity to inhibition by cycloheximide in Chlamydomonas reinhardtii.Plant Cell. 2013 Mar;25(3):985-98. doi: 10.1105/tpc.113.109256. Epub 2013 Mar 19. Plant Cell. 2013. PMID: 23512853 Free PMC article.
-
Natural variation of the amino-terminal glutamine-rich domain in Drosophila argonaute2 is not associated with developmental defects.PLoS One. 2010 Dec 17;5(12):e15264. doi: 10.1371/journal.pone.0015264. PLoS One. 2010. PMID: 21253006 Free PMC article.
-
Mutations in PpAGO3 Lead to Enhanced Virulence of Phytophthora parasitica by Activation of 25-26 nt sRNA-Associated Effector Genes.Front Microbiol. 2022 Mar 24;13:856106. doi: 10.3389/fmicb.2022.856106. eCollection 2022. Front Microbiol. 2022. PMID: 35401482 Free PMC article.
-
The evolutionary journey of Argonaute proteins.Nat Struct Mol Biol. 2014 Sep;21(9):743-53. doi: 10.1038/nsmb.2879. Nat Struct Mol Biol. 2014. PMID: 25192263 Free PMC article. Review.
-
Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution.Biol Direct. 2010 Feb 4;5:7. doi: 10.1186/1745-6150-5-7. Biol Direct. 2010. PMID: 20132544 Free PMC article.
References
-
- Farazi T. A., Juranek S. A., Tuschl T. (2008) Development 135, 1201–1214 - PubMed
-
- Tolia N. H., Joshua-Tor L. (2007) Nat. Chem. Biol. 3, 36–43 - PubMed
-
- Hutvagner G., Simard M. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 22–32 - PubMed
-
- Hammond S. M., Boettcher S., Caudy A. A., Kobayashi R., Hannon G. J. (2001) Science 293, 1146–1150 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

