A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin
- PMID: 19880608
- PMCID: PMC2798274
- DOI: 10.1128/JB.01260-09
A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin
Abstract
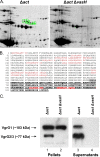
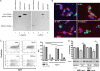
We recently delineated the importance of a type VI secretion system (T6SS) gene cluster in the virulence of diarrheal isolate SSU of Aeromonas hydrophila and showed that VasH, a sigma(54) activator and T6SS component, was involved in the production of its associated effectors, e.g., hemolysin-coregulated protein. To identify additional T6SS effectors and/or secreted proteins, we subjected culture supernatants from deletion mutants of A. hydrophila, namely, a Delta act mutant (a T2SS-associated cytotoxic enterotoxin-encoding gene) and a Delta act Delta vasH mutant, to 2-dimensional gel electrophoresis and mass spectrometric analysis. Based on these approaches, we identified a member of the VgrG protein family, VgrG1, that contained a vegetative insecticidal protein (VIP-2) domain at its carboxyl-terminal end. Consequently, the vgrG1 gene was cloned in pBI-EGFP and pET-30a vectors to be expressed in HeLa Tet-Off cells and Escherichia coli, respectively. We assessed the ADP-ribosyltransferase (ADPRT) activity of various domains of purified recombinant VgrG1 (rVgrG1) and provided evidence that only the full-length VgrG1, as well as its carboxyl-terminal domain encoding the VIP-2 domain, showed ADPRT activity. Importantly, bacterium-host cell interaction was needed for the T6SS to induce cytotoxicity in eukaryotic cells, and we demonstrated translocation of VgrG1. Furthermore, our data indicated that expression of the genes encoding the full-length VgrG1 and its carboxyl-terminal domain in HeLa Tet-Off cells disrupted the actin cytoskeleton, which was followed by a decrease in cell viability and an increase in apoptosis. Taken together, these findings demonstrated for the first time that VgrG1 of A. hydrophila possessed actin ADPRT activity associated with its VIP-2 domain and that this domain alone was able to induce a rounded phenotype in HeLa Tet-Off cells, followed by apoptosis mediated by caspase 9 activation.
Figures





References
-
- Aktories, K., M. Barmann, I. Ohishi, S. Tsuyama, K. H. Jakobs, and E. Habermann. 1986. Botulinum C2 toxin ADP-ribosylates actin. Nature 322:390-392. - PubMed
-
- Aktories, K., U. Weller, and G. S. Chhatwal. 1987. Clostridium botulinum type C produces a novel ADP-ribosyltransferase distinct from botulinum C2 toxin. FEBS Lett. 212:109-113. - PubMed
-
- Albert, M. J., M. Ansaruzzaman, K. A. Talukder, A. K. Chopra, I. Kuhn, M. Rahman, A. S. Faruque, M. S. Islam, R. B. Sack, and R. Mollby. 2000. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J. Clin. Microbiol. 38:3785-3790. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

