Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors
- PMID: 19880753
- PMCID: PMC2812070
- DOI: 10.1124/mol.109.061234
Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors
Abstract
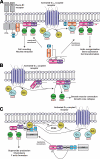
Activation of certain classes of G protein-coupled receptors (GPCRs) can lead to alterations in the actin cytoskeleton, gene transcription, cell transformation, and other processes that are known to be regulated by Rho family small-molecular-weight GTPases. Although these responses can occur indirectly via cross-talk from canonical heterotrimeric G protein cascades, it has recently been demonstrated that Dbl family Rho guanine nucleotide exchange factors (RhoGEFs) can serve as the direct downstream effectors of heterotrimeric G proteins. Heterotrimeric Galpha(12/13), Galpha(q), and Gbetagamma subunits are each now known to directly bind and regulate RhoGEFs. Atomic structures have recently been determined for several of these RhoGEFs and their G protein complexes, providing fresh insight into the molecular mechanisms of signal transduction between GPCRs and small molecular weight G proteins. This review covers what is currently known about the structure, function, and regulation of these recently recognized effectors of heterotrimeric G proteins.
Figures


References
-
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. (2000) Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102: 625–633 - PubMed
-
- Aghazadeh B, Zhu K, Kubiseski TJ, Liu GA, Pawson T, Zheng Y, Rosen MK. (1998) Structure and mutagenesis of the Dbl homology domain. Nat Struct Biol 5: 1098–1107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

