Fleshy fruit expansion and ripening are regulated by the Tomato SHATTERPROOF gene TAGL1
- PMID: 19880793
- PMCID: PMC2782289
- DOI: 10.1105/tpc.109.066936
Fleshy fruit expansion and ripening are regulated by the Tomato SHATTERPROOF gene TAGL1
Abstract
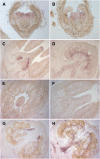
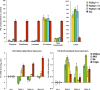
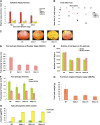
The maturation and ripening of fleshy fruits is a developmental program that synchronizes seed maturation with metabolism, rendering fruit tissues desirable to seed dispersing organisms. Through RNA interference repression, we show that Tomato AGAMOUS-LIKE1 (TAGL1), the tomato (Solanum lycopersicum) ortholog of the duplicated SHATTERPROOF (SHP) MADS box genes of Arabidopsis thaliana, is necessary for fruit ripening. Tomato plants with reduced TAGL1 mRNA produced yellow-orange fruit with reduced carotenoids and thin pericarps. These fruit are also decreased in ethylene, indicating a comprehensive inhibition of maturation mediated through reduced ACC Synthase 2 expression. Furthermore, ectopic expression of TAGL1 in tomato resulted in expansion of sepals and accumulation of lycopene, supporting the role of TAGL1 in ripening. In Arabidopsis, the duplicate SHP1 and SHP2 MADS box genes regulate the development of separation layers essential for pod shatter. Expression of TAGL1 in Arabidopsis failed to completely rescue the shp1 shp2 mutant phenotypes, indicating that TAGL1 has evolved distinct molecular functions compared with its Arabidopsis counterparts. These analyses demonstrate that TAGL1 plays an important role in regulating both fleshy fruit expansion and the ripening process that together are necessary to promote seed dispersal of fleshy fruit. From this broad perspective, SHP1/2 and TAGL1, while distinct in molecular function, regulate similar activities via their necessity for seed dispersal in Arabidopsis and tomato, respectively.
Figures






References
-
- Alba, R., et al. (2004). ESTs, cDNA microarrays, and gene expression profiling: Tools for dissecting plant physiology and development. Plant J. 39 697–714. - PubMed
-
- Ayub, R., Guis, M., Ben Amor, M., Gillot, L., Roustan, J.P., Latche, A., Bouzayen, M., and Pech, J.C. (1996). Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nat. Biotechnol. 14 862–866. - PubMed
-
- Barry, C., and Giovannoni, J. (2007). Ethylene and fruit ripening. J. Plant Growth Regul. 26 143–159.
Publication types
MeSH terms
Substances
Associated data
- GDB/XD4583
- GDB/XO4792
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

