Sfp-type 4'-phosphopantetheinyl transferase is indispensable for fungal pathogenicity
- PMID: 19880801
- PMCID: PMC2782280
- DOI: 10.1105/tpc.108.064188
Sfp-type 4'-phosphopantetheinyl transferase is indispensable for fungal pathogenicity
Abstract
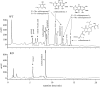
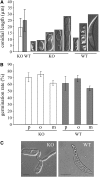
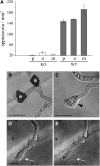
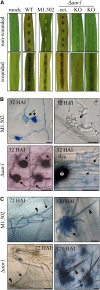
In filamentous fungi, Sfp-type 4'-phosphopantetheinyl transferases (PPTases) activate enzymes involved in primary (alpha-aminoadipate reductase [AAR]) and secondary (polyketide synthases and nonribosomal peptide synthetases) metabolism. We cloned the PPTase gene PPT1 of the maize anthracnose fungus Colletotrichum graminicola and generated PPTase-deficient mutants (Deltappt1). Deltappt1 strains were auxotrophic for Lys, unable to synthesize siderophores, hypersensitive to reactive oxygen species, and unable to synthesize polyketides (PKs). A differential analysis of secondary metabolites produced by wild-type and Deltappt1 strains led to the identification of six novel PKs. Infection-related morphogenesis was affected in Deltappt1 strains. Rarely formed appressoria of Deltappt1 strains were nonmelanized and ruptured on intact plant. The hyphae of Deltappt1 strains colonized wounded maize (Zea mays) leaves but failed to generate necrotic anthracnose disease symptoms and were defective in asexual sporulation. To analyze the pleiotropic pathogenicity phenotype, we generated AAR-deficient mutants (Deltaaar1) and employed a melanin-deficient mutant (M1.502). Results indicated that PPT1 activates enzymes required at defined stages of infection. Melanization is required for cell wall rigidity and appressorium function, and Lys supplied by the AAR1 pathway is essential for necrotrophic development. As PPTase-deficient mutants of Magnaporthe oryzea were also nonpathogenic, we conclude that PPTases represent a novel fungal pathogenicity factor.
Figures





Similar articles
-
Sfp-type 4'-phosphopantetheinyl transferase is required for lysine synthesis, tolerance to oxidative stress and virulence in the plant pathogenic fungus Cochliobolus sativus.Mol Plant Pathol. 2012 May;13(4):375-87. doi: 10.1111/j.1364-3703.2011.00756.x. Epub 2011 Oct 24. Mol Plant Pathol. 2012. PMID: 22023083 Free PMC article.
-
Virulence, Host-Selective Toxin Production, and Development of Three Cochliobolus Phytopathogens Lacking the Sfp-Type 4'-Phosphopantetheinyl Transferase Ppt1.Mol Plant Microbe Interact. 2015 Oct;28(10):1130-41. doi: 10.1094/MPMI-03-15-0068-R. Epub 2015 Oct 5. Mol Plant Microbe Interact. 2015. PMID: 26168137
-
Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola.Mol Plant Microbe Interact. 2014 Apr;27(4):315-27. doi: 10.1094/MPMI-09-13-0267-R. Mol Plant Microbe Interact. 2014. PMID: 24261846
-
Biosynthesis of secondary metabolites in the rice blast fungus Magnaporthe grisea: the role of hybrid PKS-NRPS in pathogenicity.Mycol Res. 2008 Feb;112(Pt 2):207-15. doi: 10.1016/j.mycres.2007.08.003. Epub 2007 Aug 17. Mycol Res. 2008. PMID: 18272356 Review.
-
Discovery of ML 267 as a Novel Inhibitor of Pathogenic Sfp phosphopantetheinyl transferase (PPTase).2012 Mar 21 [updated 2013 Sep 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2012 Mar 21 [updated 2013 Sep 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 24260781 Free Books & Documents. Review.
Cited by
-
Tracking Fungal Growth: Establishment of Arp1 as a Marker for Polarity Establishment and Active Hyphal Growth in Filamentous Ascomycetes.J Fungi (Basel). 2021 Jul 20;7(7):580. doi: 10.3390/jof7070580. J Fungi (Basel). 2021. PMID: 34356959 Free PMC article.
-
The Sfp-type 4'-phosphopantetheinyl transferase Ppt1 of Fusarium fujikuroi controls development, secondary metabolism and pathogenicity.PLoS One. 2012;7(5):e37519. doi: 10.1371/journal.pone.0037519. Epub 2012 May 25. PLoS One. 2012. PMID: 22662164 Free PMC article.
-
Biotrophy-specific downregulation of siderophore biosynthesis in Colletotrichum graminicola is required for modulation of immune responses of maize.Mol Microbiol. 2014 Apr;92(2):338-55. doi: 10.1111/mmi.12561. Epub 2014 Mar 17. Mol Microbiol. 2014. PMID: 24674132 Free PMC article.
-
Natural Urease Inhibitors Reduce the Severity of Disease Symptoms, Dependent on the Lifestyle of the Pathogens.J Fungi (Basel). 2023 Jun 28;9(7):708. doi: 10.3390/jof9070708. J Fungi (Basel). 2023. PMID: 37504697 Free PMC article.
-
New gene models and alternative splicing in the maize pathogen Colletotrichum graminicola revealed by RNA-Seq analysis.BMC Genomics. 2014 Oct 2;15(1):842. doi: 10.1186/1471-2164-15-842. BMC Genomics. 2014. PMID: 25281481 Free PMC article.
References
-
- Antelo, L., Hof, C., Welzel, K., Eisfeld, K., Sterner, O., and Anke, H. (2006). Siderophores produced by Magnaporthe grisea in the presence and absence of iron. Z. Naturforsch. C 61 461–464. - PubMed
-
- Baker, S.E., Kroken, S., Inderbitzin, P., Asvarak, T., Li, B.Y., Shi, L., Yoder, O.C., and Turgeon, B.G. (2006). Two polyketide synthase-encoding genes are required for biosynthesis of the polyketide virulence factor, T-toxin, by Cochliobolus heterostrophus. Mol. Plant Microbe Interact. 19 139–149. - PubMed
-
- Bechinger, C., Giebel, K.-F., Schnell, M., Leiderer, P., Deising, H.B., and Bastmeyer, M. (1999). Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science 285 1896–1899. - PubMed
-
- Becker, D.M., and Lundblad, V. (2001). Introduction of DNA into yeast cells. In Current Protocols in Molecular Biology, F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, eds (New York: John Wiley & Sons), pp. 13.17.11–13.17.10. - PubMed
-
- Bergstrom, G.C., and Nicholson, R.L. (1999). The biology of corn anthracnose. Knowledge to exploit for improved management. Plant Dis. 83 596–608. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

