T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii
- PMID: 19881508
- PMCID: PMC2803073
- DOI: 10.1038/ni.1816
T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii
Abstract
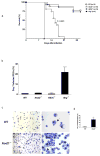
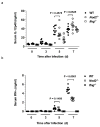
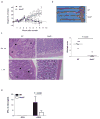
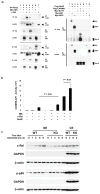
Nod2 belongs to the nucleotide-binding oligomerization domain receptor (NLR) family of proteins, which function as intracellular pathogen sensors in innate immune cells. Nod2 deficiency results in an impaired immune response to bacterial pathogens. However, how this protein promotes host defense against intracellular parasites is unknown. Here we found that Nod2(-/-) mice had less clearance of Toxoplasma gondii and lower interferon-gamma (IFN-gamma) production. Reconstitution of T cell-deficient mice with Nod2(-/-) T cells followed by T. gondii infection demonstrated a T cell-intrinsic defect. Nod2(-/-) CD4(+) T cells had poor helper T cell differentiation, which was associated with impaired production of interleukin 2 (IL-2) and nuclear accumulation of the transcription factor subunit c-Rel. Our data demonstrate a T cell-intrinsic role for Nod2 signaling that is critical for host defense against T. gondii.
Figures




Comment in
-
T cells need Nod too?Nat Immunol. 2009 Dec;10(12):1231-3. doi: 10.1038/ni1209-1231. Nat Immunol. 2009. PMID: 19915621 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

